Cell cycle arrest through indirect transcriptional repression by p53: I have a DREAM
- PMID: 29125603
- PMCID: PMC5729532
- DOI: 10.1038/cdd.2017.172
Cell cycle arrest through indirect transcriptional repression by p53: I have a DREAM
Abstract
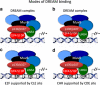
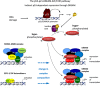
Activation of the p53 tumor suppressor can lead to cell cycle arrest. The key mechanism of p53-mediated arrest is transcriptional downregulation of many cell cycle genes. In recent years it has become evident that p53-dependent repression is controlled by the p53-p21-DREAM-E2F/CHR pathway (p53-DREAM pathway). DREAM is a transcriptional repressor that binds to E2F or CHR promoter sites. Gene regulation and deregulation by DREAM shares many mechanistic characteristics with the retinoblastoma pRB tumor suppressor that acts through E2F elements. However, because of its binding to E2F and CHR elements, DREAM regulates a larger set of target genes leading to regulatory functions distinct from pRB/E2F. The p53-DREAM pathway controls more than 250 mostly cell cycle-associated genes. The functional spectrum of these pathway targets spans from the G1 phase to the end of mitosis. Consequently, through downregulating the expression of gene products which are essential for progression through the cell cycle, the p53-DREAM pathway participates in the control of all checkpoints from DNA synthesis to cytokinesis including G1/S, G2/M and spindle assembly checkpoints. Therefore, defects in the p53-DREAM pathway contribute to a general loss of checkpoint control. Furthermore, deregulation of DREAM target genes promotes chromosomal instability and aneuploidy of cancer cells. Also, DREAM regulation is abrogated by the human papilloma virus HPV E7 protein linking the p53-DREAM pathway to carcinogenesis by HPV. Another feature of the pathway is that it downregulates many genes involved in DNA repair and telomere maintenance as well as Fanconi anemia. Importantly, when DREAM function is lost, CDK inhibitor drugs employed in cancer treatment such as Palbociclib, Abemaciclib and Ribociclib can compensate for defects in early steps in the pathway upstream from cyclin/CDK complexes. In summary, the p53-p21-DREAM-E2F/CHR pathway controls a plethora of cell cycle genes, can contribute to cell cycle arrest and is a target for cancer therapy.
Conflict of interest statement
The author declares no conflict of interest.
Figures


References
-
- Schwartz D, Rotter V. p53-dependent cell cycle control: response to genotoxic stress. Semin Cancer Biol 1998; 8: 325–336. - PubMed
-
- Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell 2009; 137: 413–431. - PubMed
-
- Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 1998; 282: 1497–1501. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

