Two Phase 1, Open-Label, Single-Dose, Randomized, Crossover Studies to Assess the Pharmacokinetics, Safety, and Tolerability of Orally Administered Granules of Secnidazole (2 g) in Healthy Female Volunteers Under Different Administration Conditions
- PMID: 29125715
- PMCID: PMC6033001
- DOI: 10.1002/cpdd.406
Two Phase 1, Open-Label, Single-Dose, Randomized, Crossover Studies to Assess the Pharmacokinetics, Safety, and Tolerability of Orally Administered Granules of Secnidazole (2 g) in Healthy Female Volunteers Under Different Administration Conditions
Abstract
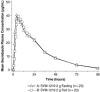
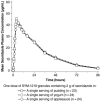
Bacterial vaginosis (BV) is the most common vaginal infection in reproductive-age women and a significant risk factor for sexually transmitted diseases and pregnancy complications. Standard 5- to 7-day antimicrobial treatments for BV are associated with high rates of recurrence and adverse events. SYM-1219 is a novel granule formulation containing 2 g of secnidazole, developed as an oral, single-dose BV treatment. Two phase 1, open-label, single-center, randomized, crossover trials (studies 102 and 103) assessed the pharmacokinetics and safety of SYM-1219 single doses (≥7-day washout between doses) in healthy, nonpregnant women aged 18 to 65 years inclusive. Study 102 compared SYM-1219 in applesauce in fasted vs fed states. Study 103 compared SYM-1219 (fasted) in pudding and yogurt vs applesauce. Studies 102 and 103 each dosed 24 subjects (mean [standard deviation] ages, 36 [1.8] and 40 [11.6] years, respectively). In both studies the 90% confidence intervals for all treatment comparisons of maximum plasma concentration, area under the concentration-time curve from 0 to last measurable concentration and to infinity, geometric mean ratios were within 80% to 125%, demonstrating bioequivalence. In both studies median fasted time to maximum plasma concentration was 4 hours (6 hours fed in study 102), and mean half-life ranged from 17 to 19 hours. Treatment-emergent adverse events occurred in 70.8% and 83.3% subjects in studies 102 and 103, respectively, most commonly headache (41.7% and 50.0%) and gastrointestinal treatment-emergent adverse events. The pharmacokinetics of SYM-1219 were similar in fed and fasted states and when administered in different foods.
Keywords: adverse events; bacterial vaginosis; pharmacokinetics; secnidazole; treatment.
© 2017 The Authors. Clinical Pharmacology in Drug Development published by Wiley Periodicals, Inc. on behalf of The American College of Clinical Pharmacology.
Figures
Similar articles
-
Effect of food on the pharmacokinetics of oxycodone and naltrexone from ALO-02, an extended release formulation of oxycodone with sequestered naltrexone.Clin Pharmacol Drug Dev. 2015 Sep;4(5):370-6. doi: 10.1002/cpdd.177. Epub 2015 Jan 22. Clin Pharmacol Drug Dev. 2015. PMID: 27137146 Clinical Trial.
-
Lack of a Pharmacokinetic Interaction Between SYM-1219 Granules Containing 2 Grams of Secnidazole and a Combined Oral Contraceptive in a Phase 1, Randomized, Open-Label Study in Healthy Female Volunteers.Adv Ther. 2017 Jan;33(12):2229-2241. doi: 10.1007/s12325-016-0411-9. Epub 2016 Oct 15. Adv Ther. 2017. PMID: 27744624 Clinical Trial.
-
A phase-3, double-blind, placebo-controlled study of the effectiveness and safety of single oral doses of secnidazole 2 g for the treatment of women with bacterial vaginosis.Am J Obstet Gynecol. 2017 Dec;217(6):678.e1-678.e9. doi: 10.1016/j.ajog.2017.08.017. Epub 2017 Sep 1. Am J Obstet Gynecol. 2017. PMID: 28867602 Clinical Trial.
-
Secnidazole for treatment of bacterial vaginosis: a systematic review.BMC Womens Health. 2019 Oct 21;19(1):121. doi: 10.1186/s12905-019-0822-2. BMC Womens Health. 2019. PMID: 31638955 Free PMC article.
-
Secnidazole. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic use in the management of protozoal infections and bacterial vaginosis.Drugs. 1996 Apr;51(4):621-38. doi: 10.2165/00003495-199651040-00007. Drugs. 1996. PMID: 8706597 Review.
Cited by
-
Secnidazole: a treatment for trichomoniasis in adolescents and adults.Expert Rev Anti Infect Ther. 2022 Aug;20(8):1067-1076. doi: 10.1080/14787210.2022.2080656. Epub 2022 Jun 8. Expert Rev Anti Infect Ther. 2022. PMID: 35642509 Free PMC article.
-
Efficacy and Safety of Single Oral Dosing of Secnidazole for Trichomoniasis in Women: Results of a Phase 3, Randomized, Double-Blind, Placebo-Controlled, Delayed-Treatment Study.Clin Infect Dis. 2021 Sep 15;73(6):e1282-e1289. doi: 10.1093/cid/ciab242. Clin Infect Dis. 2021. PMID: 33768237 Free PMC article. Clinical Trial.
-
Secnidazole for Trichomoniasis in Women and Men.Sex Med Rev. 2022 Apr;10(2):255-262. doi: 10.1016/j.sxmr.2021.12.004. Epub 2022 Feb 10. Sex Med Rev. 2022. PMID: 35153156 Free PMC article. Review.
References
-
- Centers for Disease Control and Prevention . Bacterial Vaginosis CDC Fact Sheet. Rockville MD: Division of STD Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention; 2017. https://www.cdc.gov/std/bv/stdfact-bacterial-vaginosis.htm. Accessed March 8, 2017
-
- Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001‐2004 National Health and Nutrition Examination Survey data. Obstet Gynecol. 2007;109(1):114–120. - PubMed
-
- Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001‐2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007;34(11):864–869. - PubMed
-
- Laxmi U, Agrawal S, Raghunandan C, Randhawa VS, Saili A. Association of bacterial vaginosis with adverse fetomaternal outcome in women with spontaneous preterm labor: a prospective cohort study. J Matern Fetal Neonatal Med. 2012;25(1):64–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

