Safety, Pharmacokinetics, and Immunogenicity of Obiltoxaximab After Intramuscular Administration to Healthy Humans
- PMID: 29125719
- PMCID: PMC6668011
- DOI: 10.1002/cpdd.410
Safety, Pharmacokinetics, and Immunogenicity of Obiltoxaximab After Intramuscular Administration to Healthy Humans
Abstract
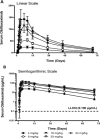
Inhalational anthrax is a highly lethal infection caused by Bacillus anthracis and a serious bioterrorism threat. Protective antigen (PA) is a critical component required for the virulence of Bacillus anthracis. Obiltoxaximab, a high-affinity monoclonal antibody that neutralizes PA, is approved in the United States for intravenous use for the treatment of inhalational anthrax in combination with appropriate antibacterial drugs and for prophylaxis of inhalational anthrax when alternative therapies are not available or appropriate. Here, we explored the safety, pharmacokinetics (PK), and immunogenicity of obiltoxaximab administered by intramuscular injection at doses of 4, 8, 16, 20, and 24 mg/kg in healthy humans. Systemic exposures were approximately dose proportional, maximum serum concentrations were observed after 6-9 days, and terminal half-life ranged from 16 to 23 days. Average absolute intramuscular bioavailability was 64%. Obiltoxaximab was well tolerated, and local tolerability was acceptable up to 24 mg/kg intramuscularly, up to 6 injections per dose, and up to 5 mL per injection. No injection-site abscesses or hypersensitivity reactions occurred; no subjects developed treatment-emergent antitherapeutic antibodies over the study period of 71 days.
Keywords: anthrax, obiltoxaximab; intramuscular; pharmacokinetics; safety.
© 2017, The American College of Clinical Pharmacology.
Figures
Similar articles
-
Obiltoxaximab Prevents Disseminated Bacillus anthracis Infection and Improves Survival during Pre- and Postexposure Prophylaxis in Animal Models of Inhalational Anthrax.Antimicrob Agents Chemother. 2016 Sep 23;60(10):5796-805. doi: 10.1128/AAC.01102-16. Print 2016 Oct. Antimicrob Agents Chemother. 2016. PMID: 27431219 Free PMC article.
-
Obiltoxaximab: First Global Approval.Drugs. 2016 May;76(7):823-30. doi: 10.1007/s40265-016-0577-0. Drugs. 2016. PMID: 27085536 Review.
-
Pharmacokinetics and Tolerability of Obiltoxaximab: A Report of 5 Healthy Volunteer Studies.Clin Ther. 2016 Sep;38(9):2083-2097.e7. doi: 10.1016/j.clinthera.2016.07.170. Epub 2016 Aug 24. Clin Ther. 2016. PMID: 27568215 Clinical Trial.
-
Obiltoxaximab: Adding to the Treatment Arsenal for Bacillus anthracis Infection.Ann Pharmacother. 2017 Oct;51(10):908-913. doi: 10.1177/1060028017713029. Epub 2017 Jun 2. Ann Pharmacother. 2017. PMID: 28573869 Review.
-
Development of Protective Immunity in New Zealand White Rabbits Challenged with Bacillus anthracis Spores and Treated with Antibiotics and Obiltoxaximab, a Monoclonal Antibody against Protective Antigen.Antimicrob Agents Chemother. 2018 Jan 25;62(2):e01590-17. doi: 10.1128/AAC.01590-17. Print 2018 Feb. Antimicrob Agents Chemother. 2018. PMID: 29133571 Free PMC article.
Cited by
-
Therapeutic monoclonal antibody treatment protects nonhuman primates from severe Venezuelan equine encephalitis virus disease after aerosol exposure.PLoS Pathog. 2019 Dec 2;15(12):e1008157. doi: 10.1371/journal.ppat.1008157. eCollection 2019 Dec. PLoS Pathog. 2019. PMID: 31790515 Free PMC article.
-
Development of a New Cell-Based AP-1 Gene Reporter Potency Assay for Anti-Anthrax Toxin Therapeutics.Toxins (Basel). 2023 Aug 28;15(9):528. doi: 10.3390/toxins15090528. Toxins (Basel). 2023. PMID: 37755954 Free PMC article.
-
Anthrax: Transmission, Pathogenesis, Prevention and Treatment.Toxins (Basel). 2025 Jan 24;17(2):56. doi: 10.3390/toxins17020056. Toxins (Basel). 2025. PMID: 39998073 Free PMC article. Review.
-
New FDA approved antibacterial drugs: 2015-2017.Discoveries (Craiova). 2018 Apr 4;6(1):e81. doi: 10.15190/d.2018.1. Discoveries (Craiova). 2018. PMID: 32309599 Free PMC article. Review.
-
A randomized, double-blind, Phase 1, single- and multiple-dose placebo-controlled study of the safety and pharmacokinetics of IN-006, an inhaled antibody treatment for COVID-19 in healthy volunteers.EBioMedicine. 2025 Mar;113:105582. doi: 10.1016/j.ebiom.2025.105582. Epub 2025 Feb 8. EBioMedicine. 2025. PMID: 39923743 Free PMC article. Clinical Trial.
References
-
- Dixon TC, Meselson M, Guillemin J, Hanna PC. Anthrax. N Engl J Med. 1999;341:815–826. - PubMed
-
- Mock M, Fouet A. Anthrax. Annu Rev Microbiol. 2001;55:647–671. - PubMed
-
- Inglesby TV, O'Toole T, Henderson DA, et al. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA. 2002;287:2236–2252. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical