Imaging PD-L1 Expression with ImmunoPET
- PMID: 29125731
- PMCID: PMC5773933
- DOI: 10.1021/acs.bioconjchem.7b00631
Imaging PD-L1 Expression with ImmunoPET
Abstract
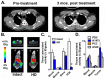
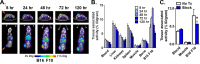
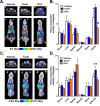
High sensitivity imaging tools could provide a more holistic view of target antigen expression to improve the identification of patients who might benefit from cancer immunotherapy. We developed for immunoPET a novel recombinant human IgG1 (termed C4) that potently binds an extracellular epitope on human and mouse PD-L1 and radiolabeled the antibody with zirconium-89. Small animal PET/CT studies showed that 89Zr-C4 detected antigen levels on a patient derived xenograft (PDX) established from a non-small-cell lung cancer (NSCLC) patient before an 8-month response to anti-PD-1 and anti-CTLA4 therapy. Importantly, the concentration of antigen is beneath the detection limit of previously developed anti-PD-L1 radiotracers, including radiolabeled atezolizumab. We also show that 89Zr-C4 can specifically detect antigen in human NSCLC and prostate cancer models endogenously expressing a broad range of PD-L1. 89Zr-C4 detects mouse PD-L1 expression changes in immunocompetent mice, suggesting that endogenous PD-1/2 will not confound human imaging. Lastly, we found that 89Zr-C4 could detect acute changes in tumor expression of PD-L1 due to standard of care chemotherapies. In summary, we present evidence that low levels of PD-L1 in clinically relevant cancer models can be imaged with immunoPET using a novel recombinant human antibody.
Conflict of interest statement
The authors declare the following competing financial interest(s): Michael J. Evans received consulting fees and owns shares in ORIC Pharmaceuticals, Inc. Michael J. Evans received research support from GE Healthcare.
Figures

Similar articles
-
Monitoring the Response of PD-L1 Expression to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Nonsmall-Cell Lung Cancer Xenografts by Immuno-PET Imaging.Mol Pharm. 2019 Aug 5;16(8):3469-3476. doi: 10.1021/acs.molpharmaceut.9b00307. Epub 2019 Jul 8. Mol Pharm. 2019. PMID: 31283253
-
Optimizing Immuno-PET Imaging of Tumor PD-L1 Expression: Pharmacokinetic, Biodistribution, and Dosimetric Comparisons of 89Zr-Labeled Anti-PD-L1 Antibody Formats.J Nucl Med. 2022 Aug;63(8):1259-1265. doi: 10.2967/jnumed.121.262967. Epub 2021 Dec 21. J Nucl Med. 2022. PMID: 34933891 Free PMC article.
-
Immuno-PET Imaging of the Programmed Cell Death-1 Ligand (PD-L1) Using a Zirconium-89 Labeled Therapeutic Antibody, Avelumab.Mol Imaging. 2019 Jan-Dec;18:1536012119829986. doi: 10.1177/1536012119829986. Mol Imaging. 2019. PMID: 31044647 Free PMC article.
-
Targeting the PD-1/PD-L1 axis in non-small cell lung cancer.Curr Probl Cancer. 2017 Mar-Apr;41(2):111-124. doi: 10.1016/j.currproblcancer.2016.12.002. Epub 2016 Dec 23. Curr Probl Cancer. 2017. PMID: 28214087 Review.
-
89Zr-Immuno-Positron Emission Tomography in Oncology: State-of-the-Art 89Zr Radiochemistry.Bioconjug Chem. 2017 Sep 20;28(9):2211-2223. doi: 10.1021/acs.bioconjchem.7b00325. Epub 2017 Aug 24. Bioconjug Chem. 2017. PMID: 28767228 Free PMC article. Review.
Cited by
-
Radionuclide-based theranostics - a promising strategy for lung cancer.Eur J Nucl Med Mol Imaging. 2023 Jul;50(8):2353-2374. doi: 10.1007/s00259-023-06174-8. Epub 2023 Mar 16. Eur J Nucl Med Mol Imaging. 2023. PMID: 36929181 Free PMC article. Review.
-
Biomarkers for Clinical Benefit of Immune Checkpoint Inhibitor Treatment-A Review From the Melanoma Perspective and Beyond.Front Immunol. 2018 Jun 28;9:1474. doi: 10.3389/fimmu.2018.01474. eCollection 2018. Front Immunol. 2018. PMID: 30002656 Free PMC article. Review.
-
PET/CT and the Response to Immunotherapy in Lung Cancer.Curr Radiopharm. 2020;13(3):177-184. doi: 10.2174/1874471013666191220105449. Curr Radiopharm. 2020. PMID: 31858908 Free PMC article. Review.
-
Ultrasound Molecular Imaging: Principles and Applications in Cardiovascular Medicine.Curr Cardiol Rep. 2019 Mar 18;21(5):30. doi: 10.1007/s11886-019-1117-9. Curr Cardiol Rep. 2019. PMID: 30887129 Review.
-
Peptide-based PET imaging agent of tumor TIGIT expression.EJNMMI Res. 2023 May 2;13(1):38. doi: 10.1186/s13550-023-00982-7. EJNMMI Res. 2023. PMID: 37129788 Free PMC article.
References
-
- Carbognin L.; Pilotto S.; Milella M.; Vaccaro V.; Brunelli M.; Calio A.; Cuppone F.; Sperduti I.; Giannarelli D.; Chilosi; et al. (2015) Differential Activity of Nivolumab, Pembrolizumab and MPDL3280A according to the Tumor Expression of Programmed Death-Ligand-1 (PD-L1): Sensitivity Analysis of Trials in Melanoma, Lung and Genitourinary Cancers. PLoS One 10, e0130142.10.1371/journal.pone.0130142. - DOI - PMC - PubMed
-
- Herbst R. S.; Soria J. C.; Kowanetz M.; Fine G. D.; Hamid O.; Gordon M. S.; Sosman J. A.; McDermott D. F.; Powderly J. D.; Gettinger S. N.; et al. (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–7. 10.1038/nature14011. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

