Design and Synthesis of Brain Penetrant Trypanocidal N-Myristoyltransferase Inhibitors
- PMID: 29125744
- PMCID: PMC5734605
- DOI: 10.1021/acs.jmedchem.7b01255
Design and Synthesis of Brain Penetrant Trypanocidal N-Myristoyltransferase Inhibitors
Abstract
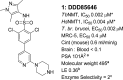
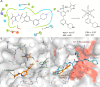
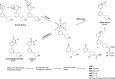
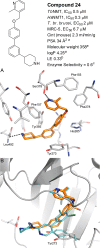
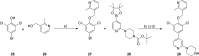
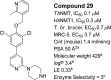
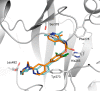
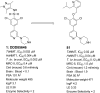
N-Myristoyltransferase (NMT) represents a promising drug target within the parasitic protozoa Trypanosoma brucei (T. brucei), the causative agent for human African trypanosomiasis (HAT) or sleeping sickness. We have previously validated T. brucei NMT as a promising druggable target for the treatment of HAT in both stages 1 and 2 of the disease. We report on the use of the previously reported DDD85646 (1) as a starting point for the design of a class of potent, brain penetrant inhibitors of T. brucei NMT.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
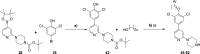
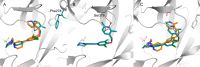
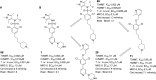
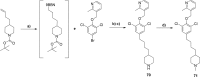
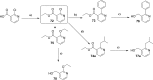
Similar articles
-
Discovery of a novel class of orally active trypanocidal N-myristoyltransferase inhibitors.J Med Chem. 2012 Jan 12;55(1):140-52. doi: 10.1021/jm201091t. Epub 2011 Dec 7. J Med Chem. 2012. PMID: 22148754 Free PMC article.
-
Lead optimization of a pyrazole sulfonamide series of Trypanosoma brucei N-myristoyltransferase inhibitors: identification and evaluation of CNS penetrant compounds as potential treatments for stage 2 human African trypanosomiasis.J Med Chem. 2014 Dec 11;57(23):9855-69. doi: 10.1021/jm500809c. Epub 2014 Nov 20. J Med Chem. 2014. PMID: 25412409 Free PMC article.
-
N-myristoyltransferase inhibitors as new leads to treat sleeping sickness.Nature. 2010 Apr 1;464(7289):728-32. doi: 10.1038/nature08893. Nature. 2010. PMID: 20360736 Free PMC article.
-
Uptake and mode of action of drugs used against sleeping sickness.Biochem Pharmacol. 2001 Jan 1;61(1):1-5. doi: 10.1016/s0006-2952(00)00477-9. Biochem Pharmacol. 2001. PMID: 11137702 Review.
-
The Inhibition of Cysteine Proteases Rhodesain and TbCatB: A Valuable Approach to Treat Human African Trypanosomiasis.Mini Rev Med Chem. 2016;16(17):1374-1391. doi: 10.2174/1389557515666160509125243. Mini Rev Med Chem. 2016. PMID: 27156518 Review.
Cited by
-
N-Myristoyltransferase as a Glycine and Lysine Myristoyltransferase in Cancer, Immunity, and Infections.ACS Chem Biol. 2020 Jul 17;15(7):1747-1758. doi: 10.1021/acschembio.0c00314. Epub 2020 Jun 10. ACS Chem Biol. 2020. PMID: 32453941 Free PMC article. Review.
-
Exploring Novel N-Myristoyltransferase Inhibitors: A Molecular Dynamics Simulation Approach.ACS Omega. 2019 Aug 15;4(9):13658-13670. doi: 10.1021/acsomega.9b00843. eCollection 2019 Aug 27. ACS Omega. 2019. PMID: 31497683 Free PMC article.
-
High-resolution snapshots of human N-myristoyltransferase in action illuminate a mechanism promoting N-terminal Lys and Gly myristoylation.Nat Commun. 2020 Feb 28;11(1):1132. doi: 10.1038/s41467-020-14847-3. Nat Commun. 2020. PMID: 32111831 Free PMC article.
-
Aminopyridines in the development of drug candidates against protozoan neglected tropical diseases.Future Med Chem. 2024 Jul 2;16(13):1357-1373. doi: 10.1080/17568919.2024.2359361. Epub 2024 Jun 10. Future Med Chem. 2024. PMID: 39109436 Free PMC article. Review.
-
N-Myristoyltransferase, a Potential Antifungal Candidate Drug-Target for Aspergillus flavus.Microbiol Spectr. 2023 Feb 14;11(1):e0421222. doi: 10.1128/spectrum.04212-22. Epub 2022 Dec 21. Microbiol Spectr. 2023. PMID: 36541770 Free PMC article.
References
-
- WHO. Trypanosomiasis, Human African trypanosomiasis (sleeping sickness). http://www.who.int/mediacentre/factsheets/fs259/en/ (accessed October 15, 2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

