Independent Photochemical Generation and Reactivity of Nitrogen-Centered Purine Nucleoside Radicals from Hydrazines
- PMID: 29125775
- PMCID: PMC5711525
- DOI: 10.1021/acs.orglett.7b03368
Independent Photochemical Generation and Reactivity of Nitrogen-Centered Purine Nucleoside Radicals from Hydrazines
Abstract
Photochemical precursors that produce dA• and dG(N2-H)• are needed to investigate their reactivity. The synthesis of two 1,1-diphenylhydrazines (1, 2) and their use as photochemical sources of dA• and dG(N2-H)• is presented. Trapping studies indicate production of these radicals with good fidelity, and 1 was incorporated into an oligonucleotide via solid-phase synthesis. Cyclic voltammetric studies show that reduction potentials of 1 and 2 are lower than those of widely used "hole sinks", e.g., 8-oxodGuo and 7-deazadGuo, to investigate DNA-hole transfer processes. These molecules could be useful (a) as sources of dA• and dG(N2-H)• at specific sites in oligonucleotides and (b) as "hole sinks" for the study of DNA-hole transfer processes.
Figures




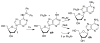
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

