Wnt/β-Catenin Signaling in Liver Development, Homeostasis, and Pathobiology
- PMID: 29125798
- PMCID: PMC5927358
- DOI: 10.1146/annurev-pathol-020117-044010
Wnt/β-Catenin Signaling in Liver Development, Homeostasis, and Pathobiology
Abstract
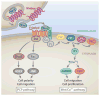
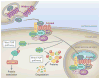
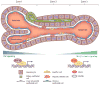
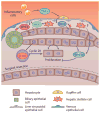
The liver is an organ that performs a multitude of functions, and its health is pertinent and indispensable to survival. Thus, the cellular and molecular machinery driving hepatic functions is of utmost relevance. The Wnt signaling pathway is one such signaling cascade that enables hepatic homeostasis and contributes to unique hepatic attributes such as metabolic zonation and regeneration. The Wnt/β-catenin pathway plays a role in almost every facet of liver biology. Furthermore, its aberrant activation is also a hallmark of various hepatic pathologies. In addition to its signaling function, β-catenin also plays a role at adherens junctions. Wnt/β-catenin signaling also influences the function of many different cell types. Due to this myriad of functions, Wnt/β-catenin signaling is complex, context-dependent, and highly regulated. In this review, we discuss the Wnt/β-catenin signaling pathway, its role in cell-cell adhesion and liver function, and the cell type-specific roles of Wnt/β-catenin signaling as it relates to liver physiology and pathobiology.
Keywords: cholangiocyte; hepatoblastoma; hepatocellular cancer; hepatocyte; liver stem cell; liver tumors; regeneration; zonation.
Figures


References
-
- Kimelman D, Xu W. β-catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25:7482–91. - PubMed
-
- Behrens J, Jerchow BA, Würtele M, Grimm J, Asbrand C, et al. Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK3β. Science. 1998;280:596–99. - PubMed
-
- Liu C, Li Y, Semenov M, Han C, Baeg GH, et al. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–47. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

