Autophagy pathway induced by a plant virus facilitates viral spread and transmission by its insect vector
- PMID: 29125860
- PMCID: PMC5708841
- DOI: 10.1371/journal.ppat.1006727
Autophagy pathway induced by a plant virus facilitates viral spread and transmission by its insect vector
Abstract
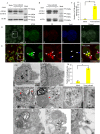
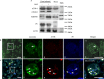
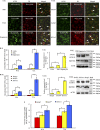
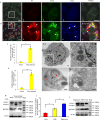
Many viral pathogens are persistently transmitted by insect vectors and cause agricultural or health problems. Generally, an insect vector can use autophagy as an intrinsic antiviral defense mechanism against viral infection. Whether viruses can evolve to exploit autophagy to promote their transmission by insect vectors is still unknown. Here, we show that the autophagic process is triggered by the persistent replication of a plant reovirus, rice gall dwarf virus (RGDV) in cultured leafhopper vector cells and in intact insects, as demonstrated by the appearance of obvious virus-containing double-membrane autophagosomes, conversion of ATG8-I to ATG8-II and increased level of autophagic flux. Such virus-containing autophagosomes seem able to mediate nonlytic viral release from cultured cells or facilitate viral spread in the leafhopper intestine. Applying the autophagy inhibitor 3-methyladenine or silencing the expression of Atg5 significantly decrease viral spread in vitro and in vivo, whereas applying the autophagy inducer rapamycin or silencing the expression of Torc1 facilitate such viral spread. Furthermore, we find that activation of autophagy facilitates efficient viral transmission, whereas inhibiting autophagy blocks viral transmission by its insect vector. Together, these results indicate a plant virus can induce the formation of autophagosomes for carrying virions, thus facilitating viral spread and transmission by its insect vector. We believe that such a role for virus-induced autophagy is common for vector-borne persistent viruses during their transmission by insect vectors.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Fibrillar structures induced by a plant reovirus target mitochondria to activate typical apoptotic response and promote viral infection in insect vectors.PLoS Pathog. 2019 Jan 17;15(1):e1007510. doi: 10.1371/journal.ppat.1007510. eCollection 2019 Jan. PLoS Pathog. 2019. PMID: 30653614 Free PMC article.
-
Filamentous Structures Induced by a Phytoreovirus Mediate Viral Release from Salivary Glands in Its Insect Vector.J Virol. 2017 May 26;91(12):e00265-17. doi: 10.1128/JVI.00265-17. Print 2017 Jun 15. J Virol. 2017. PMID: 28381575 Free PMC article.
-
Virus-induced tubule: a vehicle for rapid spread of virions through basal lamina from midgut epithelium in the insect vector.J Virol. 2014 Sep;88(18):10488-500. doi: 10.1128/JVI.01261-14. Epub 2014 Jun 25. J Virol. 2014. PMID: 24965461 Free PMC article.
-
Vector mediated transmission of persistently transmitted plant viruses.Curr Opin Virol. 2018 Feb;28:127-132. doi: 10.1016/j.coviro.2017.12.004. Epub 2018 Jan 4. Curr Opin Virol. 2018. PMID: 29306179 Review.
-
Rice Reoviruses in Insect Vectors.Annu Rev Phytopathol. 2016 Aug 4;54:99-120. doi: 10.1146/annurev-phyto-080615-095900. Epub 2016 May 25. Annu Rev Phytopathol. 2016. PMID: 27296147 Review.
Cited by
-
Molecular Mechanisms of Autophagy Regulation in Plants and Their Applications in Agriculture.Front Plant Sci. 2021 Feb 16;11:618944. doi: 10.3389/fpls.2020.618944. eCollection 2020. Front Plant Sci. 2021. PMID: 33664753 Free PMC article. Review.
-
Silencing the Autophagy-Related Genes ATG3 and ATG9 Promotes SRBSDV Propagation and Transmission in Sogatella furcifera.Insects. 2022 Apr 18;13(4):394. doi: 10.3390/insects13040394. Insects. 2022. PMID: 35447836 Free PMC article.
-
Rice Gall Dwarf Virus Promotes the Propagation and Transmission of Rice Stripe Mosaic Virus by Co-infected Insect Vectors.Front Microbiol. 2022 Feb 11;13:834712. doi: 10.3389/fmicb.2022.834712. eCollection 2022. Front Microbiol. 2022. PMID: 35222343 Free PMC article.
-
Autophagy mediates a direct synergistic interaction during co-transmission of two distinct arboviruses by insect vectors.Sci China Life Sci. 2023 Jul;66(7):1665-1681. doi: 10.1007/s11427-022-2228-y. Epub 2023 Mar 9. Sci China Life Sci. 2023. PMID: 36917406
-
Fibrillar structures induced by a plant reovirus target mitochondria to activate typical apoptotic response and promote viral infection in insect vectors.PLoS Pathog. 2019 Jan 17;15(1):e1007510. doi: 10.1371/journal.ppat.1007510. eCollection 2019 Jan. PLoS Pathog. 2019. PMID: 30653614 Free PMC article.
References
-
- Hogenhout SA, Ammar ED, Whitfield AE, Redinbaugh MG. Insect vector interactions with persistently transmitted viruses. Annu Rev Phytopathol. 2008; 46:327–59. doi: 10.1146/annurev.phyto.022508.092135 - DOI - PubMed
-
- Wei T, Li Y. Rice Reoviruses in Insect Vectors. Annu Rev Phytopathol. 2016; 54:99–120. doi: 10.1146/annurev-phyto-080615-095900 - DOI - PubMed
-
- Whitfield AE, Falk BW, Rotenberg D. Insect vector-mediated transmission of plant viruses. Virology. 2015; 479:278–89. doi: 10.1016/j.virol.2015.03.026 - DOI - PubMed
-
- Klionsky DJ, Abdelmohsen K, Abe K, Abedin MJ, Abeliovich H, Acevedo-Arozena A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016; 12(1):1–222. doi: 10.1080/15548627.2015.1100356 - DOI - PMC - PubMed
-
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008; 451(7182):1069–75. doi: 10.1038/nature06639 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

