Synaptic plasticity may underlie l-DOPA induced dyskinesia
- PMID: 29125979
- PMCID: PMC5825254
- DOI: 10.1016/j.conb.2017.10.021
Synaptic plasticity may underlie l-DOPA induced dyskinesia
Abstract
l-DOPA provides highly effective treatment for Parkinson's disease, but l-DOPA induced dyskinesia (LID) is a very debilitating response that eventually is presented by a majority of patients. A central issue in understanding the basis of LID is whether it is due to a response to chronic l-DOPA over years of therapy, and/or due to synaptic changes that follow the loss of dopaminergic neurotransmission and then triggered by acute l-DOPA administration. We review recent work that suggests that specific synaptic changes in the D1 dopamine receptor-expressing direct pathway striatal projection neurons due to loss of dopamine in Parkinson's disease are responsible for LID. Chronic l-DOPA may nevertheless modulate LID through priming mechanisms.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Authors disclose no conflict of interest.
Figures
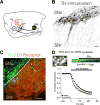
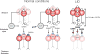
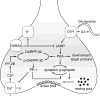
Similar articles
-
Selective loss of bi-directional synaptic plasticity in the direct and indirect striatal output pathways accompanies generation of parkinsonism and l-DOPA induced dyskinesia in mouse models.Neurobiol Dis. 2014 Nov;71:334-44. doi: 10.1016/j.nbd.2014.08.006. Epub 2014 Aug 27. Neurobiol Dis. 2014. PMID: 25171793 Free PMC article.
-
l-DOPA-induced dyskinesia is associated with a deficient numerical downregulation of striatal tyrosine hydroxylase mRNA-expressing neurons.Neuroscience. 2016 Sep 7;331:120-33. doi: 10.1016/j.neuroscience.2016.06.017. Epub 2016 Jun 16. Neuroscience. 2016. PMID: 27320210
-
Striatal tyrosine hydroxylase-positive neurons are associated with L-DOPA-induced dyskinesia in hemiparkinsonian mice.Neuroscience. 2015 Jul 9;298:302-17. doi: 10.1016/j.neuroscience.2015.04.021. Epub 2015 Apr 16. Neuroscience. 2015. PMID: 25892702
-
Striatal plasticity in Parkinson's disease and L-dopa induced dyskinesia.Parkinsonism Relat Disord. 2012 Jan;18 Suppl 1:S123-5. doi: 10.1016/S1353-8020(11)70038-4. Parkinsonism Relat Disord. 2012. PMID: 22166408 Review.
-
[Development of dyskinesias induced by treatment for Parkinson's disease: potential role of first exposure to L-DOPA (or phenomenon of priming)].Rev Neurol (Paris). 2000 Mar;156(3):224-35. Rev Neurol (Paris). 2000. PMID: 10740093 Review. French.
Cited by
-
The Rodent Models of Dyskinesia and Their Behavioral Assessment.Front Neurol. 2019 Oct 11;10:1016. doi: 10.3389/fneur.2019.01016. eCollection 2019. Front Neurol. 2019. PMID: 31681132 Free PMC article. Review.
-
Overcoming barriers to effective management of tardive dyskinesia.Neuropsychiatr Dis Treat. 2019 Apr 4;15:785-794. doi: 10.2147/NDT.S196541. eCollection 2019. Neuropsychiatr Dis Treat. 2019. PMID: 31040678 Free PMC article. Review.
-
Effects of Aging on Levo-Dihydroxyphenylalanine- Induced Dyskinesia in a Rat Model of Parkinson's Disease.Front Aging Neurosci. 2021 May 13;13:650350. doi: 10.3389/fnagi.2021.650350. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34054505 Free PMC article.
-
Translational profiling reveals novel gene expression changes in the direct and indirect pathways in a mouse model of levodopa induced dyskinesia.Front Cell Neurosci. 2025 Mar 12;18:1477511. doi: 10.3389/fncel.2024.1477511. eCollection 2024. Front Cell Neurosci. 2025. PMID: 40144773 Free PMC article.
-
Dopamine D3 receptor: A neglected participant in Parkinson Disease pathogenesis and treatment?Ageing Res Rev. 2020 Jan;57:100994. doi: 10.1016/j.arr.2019.100994. Epub 2019 Nov 22. Ageing Res Rev. 2020. PMID: 31765822 Free PMC article. Review.
References
-
- Aquino CC, Fox SH. Clinical spectrum of levodopa-induced complications. Mov Disord. 2015;30:80–89. - PubMed
-
- Bastide MF, Meissner WG, Picconi B, Fasano S, Fernagut PO, Feyder M, Francardo V, Alcacer C, Ding Y, Brambilla R, et al. Pathophysiology of L-dopa-induced motor and non-motor complications in Parkinson’s disease. Prog Neurobiol. 2015;132:96–168. - PubMed
-
- Jenner P. Molecular mechanisms of L-DOPA-induced dyskinesia. Nat Rev Neurosci. 2008;9:665–677. - PubMed
-
- Iravani MM, McCreary AC, Jenner P. Striatal plasticity in Parkinson’s disease and L-dopa induced dyskinesia. Parkinsonism Relat Disord. 2012;18(Suppl 1):S123–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

