Gating of visual processing by physiological need
- PMID: 29125986
- PMCID: PMC5889964
- DOI: 10.1016/j.conb.2017.10.020
Gating of visual processing by physiological need
Abstract
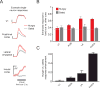
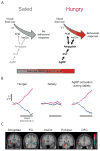
Physiological need states and associated motivational drives can bias visual processing of cues that help meet these needs. Human neuroimaging studies consistently show a hunger-dependent, selective enhancement of responses to images of food in association cortex and amygdala. More recently, cellular-resolution imaging combined with circuit mapping experiments in behaving mice have revealed underlying neuronal population dynamics and enabled tracing of pathways by which hunger circuits influence the assignment of value to visual objects in visual association cortex, insular cortex, and amygdala. These experiments begin to provide a mechanistic understanding of motivation-specific neural processing of need-relevant cues in healthy humans and in disease states such as obesity and other eating disorders.
Published by Elsevier Ltd.
Conflict of interest statement
Conflicts of interest: none
The authors have no conflicts of interest to disclose
Figures

References
-
- Castellanos EH, Charboneau E, Dietrich MS, Park S, Bradley BP, Mogg K, Cowan RL. Obese adults have visual attention bias for food cue images: evidence for altered reward system function. Int J Obes (Lond) 2009;33:1063–1073. - PubMed
-
- Loeber S, Grosshans M, Herpertz S, Kiefer F, Herpertz SC. Hunger modulates behavioral disinhibition and attention allocation to food-associated cues in normal-weight controls. Appetite. 2013;71:32–39. - PubMed
-
- Piech RM, Pastorino MT, Zald DH. All I saw was the cake. Hunger effects on attentional capture by visual food cues. Appetite. 2010;54:579–582. - PubMed
-
- Cornier MA, Von Kaenel SS, Bessesen DH, Tregellas JR. Effects of overfeeding on the neuronal response to visual food cues. Am J Clin Nutr. 2007;86:965–971. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

