α-Lipoic acid improves abnormal behavior by mitigation of oxidative stress, inflammation, ferroptosis, and tauopathy in P301S Tau transgenic mice
- PMID: 29126071
- PMCID: PMC5684493
- DOI: 10.1016/j.redox.2017.11.001
α-Lipoic acid improves abnormal behavior by mitigation of oxidative stress, inflammation, ferroptosis, and tauopathy in P301S Tau transgenic mice
Abstract
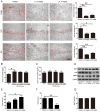
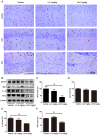
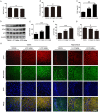
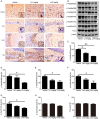
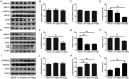
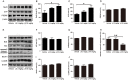
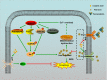
Alzheimer's disease (AD) is the most common neurodegenerative disease and is characterized by neurofibrillary tangles (NFTs) composed of Tau protein. α-Lipoic acid (LA) has been found to stabilize the cognitive function of AD patients, and animal study findings have confirmed its anti-amyloidogenic properties. However, the underlying mechanisms remain unclear, especially with respect to the ability of LA to control Tau pathology and neuronal damage. Here, we found that LA supplementation effectively inhibited the hyperphosphorylation of Tau at several AD-related sites, accompanied by reduced cognitive decline in P301S Tau transgenic mice. Furthermore, we found that LA not only inhibited the activity of calpain1, which has been associated with tauopathy development and neurodegeneration via modulating the activity of several kinases, but also significantly decreased the calcium content of brain tissue in LA-treated mice. Next, we screened for various modes of neural cell death in the brain tissue of LA-treated mice. We found that caspase-dependent apoptosis was potently inhibited, whereas autophagy did not show significant changes after LA supplementation. Interestingly, Tau-induced iron overload, lipid peroxidation, and inflammation, which are involved in ferroptosis, were significantly blocked by LA administration. These results provide compelling evidence that LA plays a role in inhibiting Tau hyperphosphorylation and neuronal loss, including ferroptosis, through several pathways, suggesting that LA may be a potential therapy for tauopathies.
Keywords: Alzheimer's disease; Ferroptosis; Oxidative stress; Tau; α-Lipoic acid.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Yoshiyama Y., Higuchi M., Zhang B., Huang S.M., Iwata N., Saido T.C., Maeda J., Suhara T., Trojanowski J.Q., Lee V.M. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53(3):337–351. - PubMed
-
- Lee V.M., Goedert M., Trojanowski J.Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001;24:1121–1159. - PubMed
-
- DeVos S.L., Miller R.L., Schoch K.M., Holmes B.B., Kebodeaux C.S., Wegener A.J., Chen G., Shen T., Tran H., Nichols B., Zanardi T.A., Kordasiewicz H.B., Swayze E.E., Bennett C.F., Diamond M.I., Miller T.M. Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci. Transl. Med. 2017;9(374) - PMC - PubMed
-
- Holtzman D.M., Carrillo M.C., Hendrix J.A., Bain L.J., Catafau A.M., Gault L.M., Goedert M., Mandelkow E., Mandelkow E.M., Miller D.S., Ostrowitzki S., Polydoro M., Smith S., Wittmann M., Hutton M. Tau: from research to clinical development. Alzheimer's & dementia. J. Alzheimer's Assoc. 2016;12(10):1033–1039. - PubMed
-
- Perez M., Cuadros R., Smith M.A., Perry G., Avila J. Phosphorylated, but not native, tau protein assembles following reaction with the lipid peroxidation product, 4-hydroxy-2-nonenal. FEBS Lett. 2000;486(3):270–274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

