Changes in total and differential leukocyte counts during the clinically silent liver phase in a controlled human malaria infection in malaria-naïve Dutch volunteers
- PMID: 29126422
- PMCID: PMC5681833
- DOI: 10.1186/s12936-017-2108-1
Changes in total and differential leukocyte counts during the clinically silent liver phase in a controlled human malaria infection in malaria-naïve Dutch volunteers
Abstract
Background: Both in endemic countries and in imported malaria, changes in total and differential leukocyte count during Plasmodium falciparum infection have been described. To study the exact dynamics of differential leukocyte counts and their ratios, they were monitored in a group of healthy non-immune volunteers in two separate Controlled Human Malaria Infection (CHMI) studies.
Methods: In two CHMI trials, CHMI-a and CHMI-b, 15 and 24 healthy malaria-naïve volunteers, respectively, were exposed to bites of infected mosquitoes, using the P. falciparum research strain NF54 and the novel clones NF135.C10 and NF166.C8. After mosquito bite exposure, twice-daily blood draws were taken to detect parasitaemia and to monitor the total and differential leukocyte counts. All subjects received a course of atovaquone-proguanil when meeting the treatment criteria.
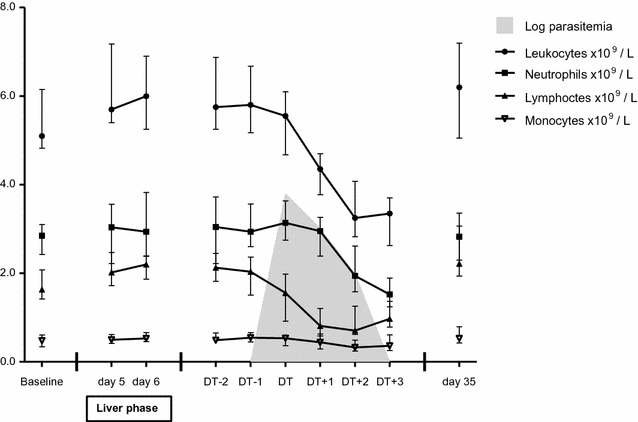
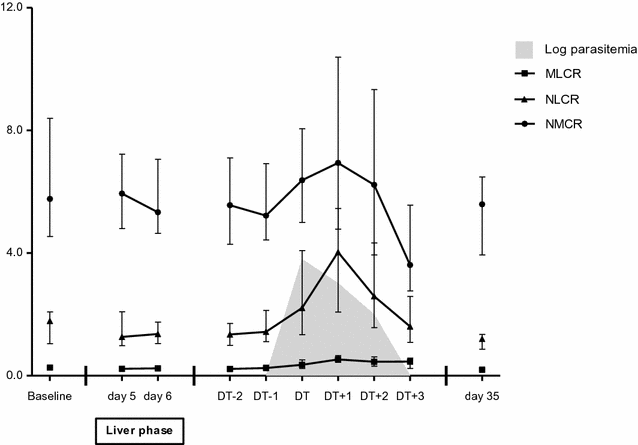
Results: A total of 39 volunteers participated in the two trials. Thirty-five participants, all 15 participants in CHMI-a and 20 of the 24 volunteers in CHMI-b, developed parasitaemia. During liver stage development of the parasite, the median total leukocyte count increased from 5.5 to 6.1 × 109 leukocytes/L (p = 0.005), the median lymphocyte count from 1.9 to 2.2 (p = 0.001) and the monocyte count from 0.50 to 0.54 (p = 0.038). During the subsequent blood stage infection, significant changes in total and differential leukocyte counts lead to a leukocytopenia (nadir median 3.3 × 109 leukocytes/L, p = 0.0001), lymphocytopenia (nadir median 0.7 × 109 lymphocytes/L, p = 0.0001) and a borderline neutropenia (nadir median 1.5 × 109 neutrophils/L, p = 0.0001). The neutrophil to lymphocyte count ratio (NLCR) reached a maximum of 4.0. Significant correlations were found between parasite load and absolute lymphocyte count (p < 0.001, correlation coefficient - 0.46) and between parasite load and NLCR (p < 0.001, correlation coefficient 0.50). All parameters normalized after parasite clearance.
Conclusions: During the clinically silent liver phase of malaria, an increase of peripheral total leukocyte count and differential lymphocytes and monocytes occurs. This finding has not been described previously. This increase is followed by the appearance of parasites in the peripheral blood after 2-3 days, accompanied by a marked decrease in total leukocyte count, lymphocyte count and the neutrophil count and a rise of the NLCR.
Keywords: Controlled Human Malaria Infection; Leukocyte count; Liver phase; Lymphocyte count; Lymphocytopenia; Monocytes; Neutropenia; Neutrophil to lymphocyte count ratio; Plasmodium falciparum.
Figures
Similar articles
-
DSM265 for Plasmodium falciparum chemoprophylaxis: a randomised, double blinded, phase 1 trial with controlled human malaria infection.Lancet Infect Dis. 2017 Jun;17(6):636-644. doi: 10.1016/S1473-3099(17)30139-1. Epub 2017 Mar 28. Lancet Infect Dis. 2017. PMID: 28363637 Free PMC article. Clinical Trial.
-
A randomized feasibility trial comparing four antimalarial drug regimens to induce Plasmodium falciparum gametocytemia in the controlled human malaria infection model.Elife. 2018 Feb 27;7:e31549. doi: 10.7554/eLife.31549. Elife. 2018. PMID: 29482720 Free PMC article. Clinical Trial.
-
Controlled Human Malaria Infection with Graded Numbers of Plasmodium falciparum NF135.C10- or NF166.C8-Infected Mosquitoes.Am J Trop Med Hyg. 2018 Sep;99(3):709-712. doi: 10.4269/ajtmh.18-0194. Epub 2018 Jul 12. Am J Trop Med Hyg. 2018. PMID: 30014816 Free PMC article. Clinical Trial.
-
Atovaquone and proguanil hydrochloride for prophylaxis of malaria.J Travel Med. 1999 May;6 Suppl 1:S21-7. J Travel Med. 1999. PMID: 23573549 Review.
-
Atovaquone and proguanil hydrochloride for treatment of malaria.J Travel Med. 1999 May;6 Suppl 1:S18-20. J Travel Med. 1999. PMID: 23573548 Review.
Cited by
-
Hematological profiles of malaria-infected patients in an endemic area of Peru.Rev Peru Med Exp Salud Publica. 2022 Jul-Sep;39(3):336-344. doi: 10.17843/rpmesp.2022.393.11908. Epub 2022 Dec 5. Rev Peru Med Exp Salud Publica. 2022. PMID: 36478167 Free PMC article.
-
Elevated Inflammation Associated with Markers of Neutrophil Function and Gastrointestinal Disruption in Pilot Study of Plasmodium fragile Co-Infection of ART-Treated SIVmac239+ Rhesus Macaques.Viruses. 2024 Jun 27;16(7):1036. doi: 10.3390/v16071036. Viruses. 2024. PMID: 39066199 Free PMC article.
-
The role of the liver in the migration of parasites of global significance.Parasit Vectors. 2019 Nov 8;12(1):531. doi: 10.1186/s13071-019-3791-2. Parasit Vectors. 2019. PMID: 31703729 Free PMC article. Review.
-
Potential Benefits of Lycopene Consumption: Rationale for Using It as an Adjuvant Treatment for Malaria Patients and in Several Diseases.Nutrients. 2022 Dec 14;14(24):5303. doi: 10.3390/nu14245303. Nutrients. 2022. PMID: 36558462 Free PMC article. Review.
-
The Rough Guide to Monocytes in Malaria Infection.Front Immunol. 2018 Dec 7;9:2888. doi: 10.3389/fimmu.2018.02888. eCollection 2018. Front Immunol. 2018. PMID: 30581439 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

