Lack of chronic neuroinflammation in the absence of focal hemorrhage in a rat model of low-energy blast-induced TBI
- PMID: 29126430
- PMCID: PMC6389215
- DOI: 10.1186/s40478-017-0483-z
Lack of chronic neuroinflammation in the absence of focal hemorrhage in a rat model of low-energy blast-induced TBI
Abstract
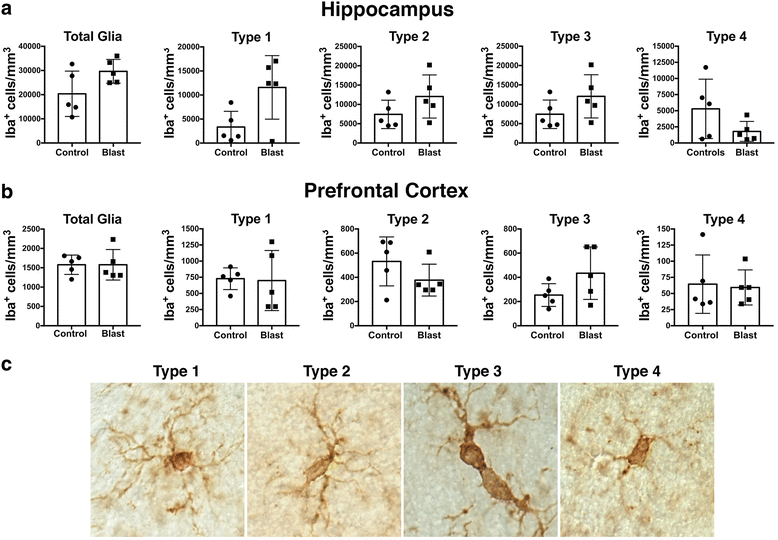
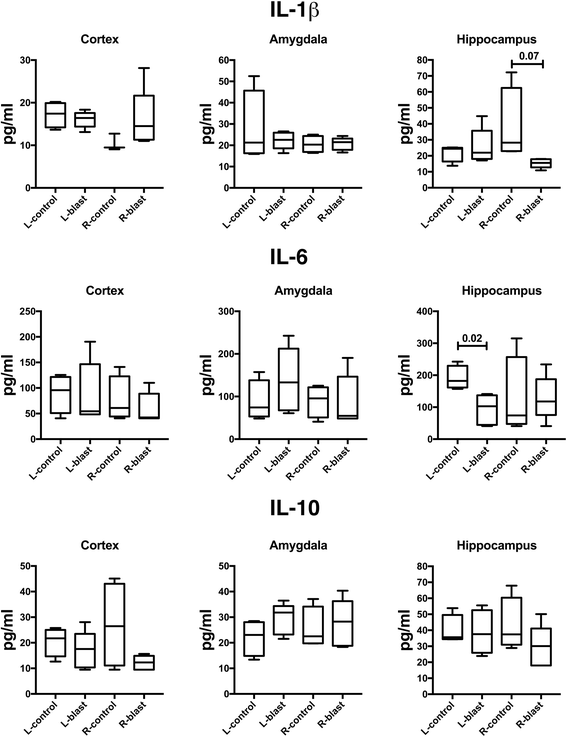
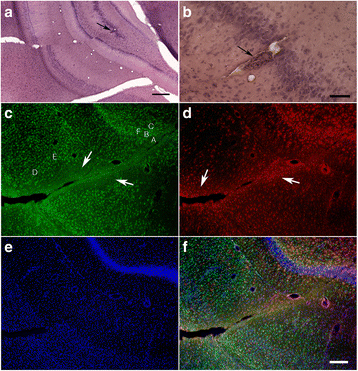
Blast-related traumatic brain injury (TBI) has been a common cause of injury in the recent conflicts in Iraq and Afghanistan. Blast waves can damage blood vessels, neurons, and glial cells within the brain. Acutely, depending on the blast energy, blast wave duration, and number of exposures, blast waves disrupt the blood-brain barrier, triggering microglial activation and neuroinflammation. Recently, there has been much interest in the role that ongoing neuroinflammation may play in the chronic effects of TBI. Here, we investigated whether chronic neuroinflammation is present in a rat model of repetitive low-energy blast exposure. Six weeks after three 74.5-kPa blast exposures, and in the absence of hemorrhage, no significant alteration in the level of microglia activation was found. At 6 weeks after blast exposure, plasma levels of fractalkine, interleukin-1β, lipopolysaccharide-inducible CXC chemokine, macrophage inflammatory protein 1α, and vascular endothelial growth factor were decreased. However, no differences in cytokine levels were detected between blast-exposed and control rats at 40 weeks. In brain, isolated changes were seen in levels of selected cytokines at 6 weeks following blast exposure, but none of these changes was found in both hemispheres or at 40 weeks after blast exposure. Notably, one animal with a focal hemorrhagic tear showed chronic microglial activation around the lesion 16 weeks post-blast exposure. These findings suggest that focal hemorrhage can trigger chronic focal neuroinflammation following blast-induced TBI, but that in the absence of hemorrhage, chronic neuroinflammation is not a general feature of low-level blast injury.
Conflict of interest statement
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury.J Neuroinflammation. 2017 Mar 15;14(1):47. doi: 10.1186/s12974-017-0819-4. J Neuroinflammation. 2017. PMID: 28292310 Free PMC article.
-
Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-κB pathway following experimental traumatic brain injury.J Neuroinflammation. 2017 Jul 24;14(1):143. doi: 10.1186/s12974-017-0917-3. J Neuroinflammation. 2017. PMID: 28738820 Free PMC article.
-
Traumatic brain injury-induced neuronal damage in the somatosensory cortex causes formation of rod-shaped microglia that promote astrogliosis and persistent neuroinflammation.Glia. 2018 Dec;66(12):2719-2736. doi: 10.1002/glia.23523. Epub 2018 Oct 30. Glia. 2018. PMID: 30378170 Free PMC article.
-
Vascular and inflammatory factors in the pathophysiology of blast-induced brain injury.Front Neurol. 2015 Mar 16;6:48. doi: 10.3389/fneur.2015.00048. eCollection 2015. Front Neurol. 2015. PMID: 25852632 Free PMC article. Review.
-
Cellular players that shape evolving pathology and neurodegeneration following traumatic brain injury.Brain Behav Immun. 2018 Jul;71:9-17. doi: 10.1016/j.bbi.2018.03.033. Epub 2018 Mar 27. Brain Behav Immun. 2018. PMID: 29601944 Review.
Cited by
-
Calcineurin signaling as a target for the treatment of alcohol abuse and neuroinflammatory disorders.Prog Mol Biol Transl Sci. 2019;167:125-142. doi: 10.1016/bs.pmbts.2019.06.008. Epub 2019 Aug 12. Prog Mol Biol Transl Sci. 2019. PMID: 31601401 Free PMC article. Review.
-
(2R,6R)-Hydroxynorketamine Treatment of Rats Exposed to Repetitive Low-Level Blast Injury.Neurotrauma Rep. 2023 Mar 30;4(1):197-217. doi: 10.1089/neur.2022.0088. eCollection 2023. Neurotrauma Rep. 2023. PMID: 37020715 Free PMC article.
-
The Neurovascular Unit as a Locus of Injury in Low-Level Blast-Induced Neurotrauma.Int J Mol Sci. 2024 Jan 17;25(2):1150. doi: 10.3390/ijms25021150. Int J Mol Sci. 2024. PMID: 38256223 Free PMC article. Review.
-
Explosive-driven double-blast exposure: molecular, histopathological, and behavioral consequences.Sci Rep. 2020 Oct 15;10(1):17446. doi: 10.1038/s41598-020-74296-2. Sci Rep. 2020. PMID: 33060648 Free PMC article.
-
Expression of GFAP and Tau Following Blast Exposure in the Cerebral Cortex of Ferrets.J Neuropathol Exp Neurol. 2021 Jan 20;80(2):112-128. doi: 10.1093/jnen/nlaa157. J Neuropathol Exp Neurol. 2021. PMID: 33421075 Free PMC article.
References
-
- Ahlers ST, Vasserman-Stokes E, Shaughness MC, Hall AA, Shear DA, Chavko M, McCarron RM, Stone JR. Assessment of the effects of acute and repeated exposure to blast overpressure in rodents: toward a greater understanding of blast and the potential ramifications for injury in humans exposed to blast. Front Neurol. 2012;3:32. doi: 10.3389/fneur.2012.00032. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

