Whole blood microRNA expression pattern differentiates patients with rheumatoid arthritis, their seropositive first-degree relatives, and healthy unrelated control subjects
- PMID: 29126434
- PMCID: PMC5681796
- DOI: 10.1186/s13075-017-1459-x
Whole blood microRNA expression pattern differentiates patients with rheumatoid arthritis, their seropositive first-degree relatives, and healthy unrelated control subjects
Abstract
Background: Epigenetic mechanisms can integrate gene-environment interactions that mediate disease transition from preclinical to clinically overt rheumatoid arthritis (RA). To better understand their role, we evaluated microRNA (miRNA, miR) expression profile in indigenous North American patients with RA who were positive for anticitrullinated protein antibodies; their autoantibody-positive, asymptomatic first-degree relatives (FDRs); and disease-free healthy control subjects (HCs).
Methods: Total RNA was isolated from whole blood samples obtained from HC (n = 12), patients with RA (n = 18), and FDRs (n = 12). Expression of 35 selected relevant miRNAs, as well as associated downstream messenger RNA (mRNA) targets of miR-103a-3p, was determined by qRT-PCR.
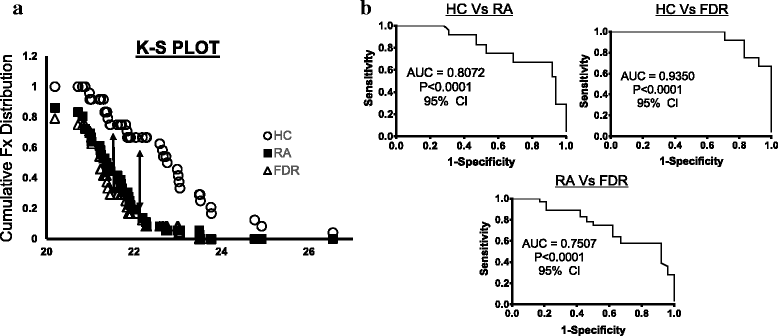
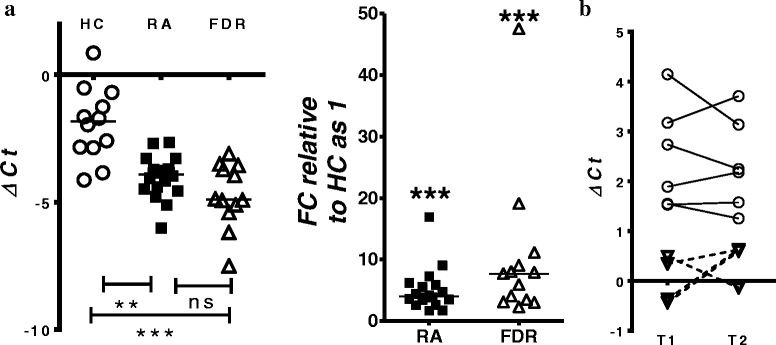
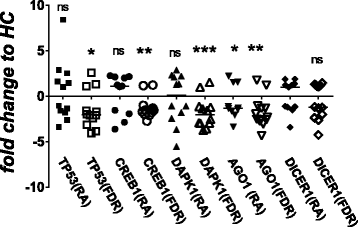
Results: Whole blood expression profiling identified significantly differential miRNA expression in patients with RA (13 miRNAs) and FDRs (10 miRNAs) compared with HCs. Among these, expression of miR-103a-3p, miR-155, miR-146a-5p, and miR-26b-3p was significantly upregulated, whereas miR-346 was significantly downregulated, in both study groups. Expression of miR-103a-3p was consistently elevated in FDRs at two time points 1 year apart. We also confirmed increased miR-103a-3p expression in peripheral blood mononuclear cells from patients with RA compared with HCs. Predicted target analyses of differentially expressed miRNAs in patients with RA and FDRs showed overlapping biological networks. Consistent with these curated networks, mRNA expression of DICER1, AGO1, CREB1, DAPK1, and TP53 was downregulated significantly with miR-103a-3p expression in FDRs.
Conclusions: We highlight systematically altered circulating miRNA expression in at-risk FDRs prior to RA onset, a profile they shared with patients with RA. Prominently consistent miR-103a-3p expression indicates its utility as a prognostic biomarker for preclinical RA while highlighting biological pathways important for transition to clinically detectable disease.
Keywords: Epigenetics; MicroRNA; Rheumatoid arthritis; Whole blood; miR-103a; miR-103a-3p; miRNA.
Conflict of interest statement
Ethics approval and consent to participate
The biomedical research ethics board of the University of Manitoba approved the overall design of the study and consent forms (ethics, 2005:093; protocol, HS14453). Specific research agreements with the study communities were developed and approved by the community leadership. The conduct of the study was guided by the principles of community-based participatory research, a cornerstone of the Canadian Institutes of Health Research guidelines for aboriginal health research (
Consent for publication
Written consent was obtained from all the participants and authors of this study.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Dysregulated microRNA expression in rheumatoid arthritis families-a comparison between rheumatoid arthritis patients, their first-degree relatives, and healthy controls.Clin Rheumatol. 2021 Jun;40(6):2387-2394. doi: 10.1007/s10067-020-05502-9. Epub 2020 Nov 18. Clin Rheumatol. 2021. PMID: 33210166 Free PMC article.
-
MiR-5571-3p and miR-135b-5p, derived from analyses of microRNA profile sequencing, correlate with increased disease risk and activity of rheumatoid arthritis.Clin Rheumatol. 2019 Jun;38(6):1753-1765. doi: 10.1007/s10067-018-04417-w. Epub 2019 Feb 1. Clin Rheumatol. 2019. PMID: 30707326
-
Utility of Select Plasma MicroRNA for Disease and Cardiovascular Risk Assessment in Patients with Rheumatoid Arthritis.J Rheumatol. 2015 Oct;42(10):1746-1751. doi: 10.3899/jrheum.150232. Epub 2015 Aug 1. J Rheumatol. 2015. PMID: 26233505 Free PMC article.
-
MicroRNAs in rheumatoid arthritis: From pathogenesis to clinical impact.Autoimmun Rev. 2019 Nov;18(11):102391. doi: 10.1016/j.autrev.2019.102391. Epub 2019 Sep 11. Autoimmun Rev. 2019. PMID: 31520804 Review.
-
MicroRNAs in rheumatoid arthritis: altered expression and diagnostic potential.Autoimmun Rev. 2015 Nov;14(11):1029-37. doi: 10.1016/j.autrev.2015.07.005. Epub 2015 Jul 8. Autoimmun Rev. 2015. PMID: 26164649 Review.
Cited by
-
Circulating levels of free 25(OH)D increase at the onset of rheumatoid arthritis.PLoS One. 2019 Sep 26;14(9):e0219109. doi: 10.1371/journal.pone.0219109. eCollection 2019. PLoS One. 2019. PMID: 31557191 Free PMC article.
-
Biomarkers (mRNAs and non-coding RNAs) for the diagnosis and prognosis of rheumatoid arthritis.Front Immunol. 2023 Feb 1;14:1087925. doi: 10.3389/fimmu.2023.1087925. eCollection 2023. Front Immunol. 2023. PMID: 36817438 Free PMC article. Review.
-
Exploring the Extracellular Vesicle MicroRNA Expression Repertoire in Patients with Rheumatoid Arthritis and Ankylosing Spondylitis Treated with TNF Inhibitors.Dis Markers. 2021 Sep 30;2021:2924935. doi: 10.1155/2021/2924935. eCollection 2021. Dis Markers. 2021. PMID: 34691284 Free PMC article.
-
miRNAs as Therapeutic Tools in Alzheimer's Disease.Int J Mol Sci. 2021 Dec 1;22(23):13012. doi: 10.3390/ijms222313012. Int J Mol Sci. 2021. PMID: 34884818 Free PMC article. Review.
-
Regulatory Non-coding RNAs for Death Associated Protein Kinase Family.Front Mol Biosci. 2021 Aug 6;8:649100. doi: 10.3389/fmolb.2021.649100. eCollection 2021. Front Mol Biosci. 2021. PMID: 34422899 Free PMC article. Review.
References
-
- Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, Habibuw MR, Vandenbroucke JP, Dijkmans BA. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50(2):380–6. doi: 10.1002/art.20018. - DOI - PubMed
-
- Sokolove J, Bromberg R, Deane KD, Lahey LJ, Derber LA, Chandra PE, Edison JD, Gilliland WR, Tibshirani RJ, Norris JM, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One. 2012;7(5):e35296. doi: 10.1371/journal.pone.0035296. - DOI - PMC - PubMed
-
- El-Gabalawy HS, Robinson DB, Hart D, Elias B, Markland J, Peschken CA, Smolik I, Montes-Aldana G, Schroeder M, Fritzler MJ, et al. Immunogenetic risks of anti-cyclical citrullinated peptide antibodies in a North American Native population with rheumatoid arthritis and their first-degree relatives. J Rheumatol. 2009;36(6):1130–5. doi: 10.3899/jrheum.080855. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

