Activity of nucleic acid polymers in rodent models of HBV infection
- PMID: 29126900
- PMCID: PMC5743593
- DOI: 10.1016/j.antiviral.2017.10.022
Activity of nucleic acid polymers in rodent models of HBV infection
Abstract
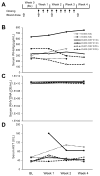
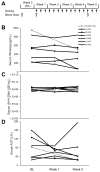
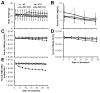
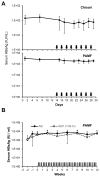
Nucleic acid polymers (NAPs) block the release of HBsAg from infected hepatocytes. These compounds have been previously shown to have the unique ability to eliminate serum surface antigen in DHBV-infected Pekin ducks and achieve multilog reduction of HBsAg or HBsAg loss in patients with chronic HBV infection and HBV/HDV coinfection. In ducks and humans, the blockage of HBsAg release by NAPs occurs by the selective targeting of the assembly and/or secretion of subviral particles (SVPs). The clinically active NAP species REP 2055 and REP 2139 were investigated in other relevant animal models of HBV infection including woodchucks chronically infected with WHV, HBV transgenic mice and HBV infected SCID-Hu mice. The liver accumulation of REP 2139 in woodchucks following subcutaneous administration was examined and was found to be similar to that observed in mice and ducks. However, in woodchucks, NAP treatment was associated with only mild (36-79% relative to baseline) reductions in WHsAg (4/10 animals) after 3-5 weeks of treatment without changes in serum WHV DNA. In HBV infected SCID-Hu mice, REP 2055 treatment was not associated with any reduction of HBsAg, HBeAg or HBV DNA in the serum after 28 days of treatment. In HBV transgenic mice, no reductions in serum HBsAg were observed with REP 2139 with up to 12 weeks of treatment. In conclusion, the antiviral effects of NAPs in DHBV infected ducks and patients with chronic HBV infection were weak or absent in woodchuck and mouse models despite similar liver accumulation of NAPs in all these species, suggesting that the mechanisms of SVP assembly and or secretion present in rodent models differs from that in DHBV and chronic HBV infections.
Keywords: HBV; HBsAg; Mouse; Nucleic acid polymer; Woodchuck.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

Similar articles
-
REP 2139: Antiviral Mechanisms and Applications in Achieving Functional Control of HBV and HDV Infection.ACS Infect Dis. 2019 May 10;5(5):675-687. doi: 10.1021/acsinfecdis.8b00156. Epub 2018 Oct 5. ACS Infect Dis. 2019. PMID: 30199230 Review.
-
Inhibition of HBsAg secretion by nucleic acid polymers in HepG2.2.15 cells.Antiviral Res. 2019 Apr;164:97-105. doi: 10.1016/j.antiviral.2019.02.009. Epub 2019 Feb 13. Antiviral Res. 2019. PMID: 30771404
-
Characterization of the antiviral effects of REP 2139 on the HBV lifecycle in vitro.Antiviral Res. 2020 Nov;183:104853. doi: 10.1016/j.antiviral.2020.104853. Epub 2020 Jun 23. Antiviral Res. 2020. PMID: 32585322
-
HBsAg isoform dynamics during NAP-based therapy of HBeAg-negative chronic HBV and HBV/HDV infection.Hepatol Commun. 2022 Aug;6(8):1870-1880. doi: 10.1002/hep4.1951. Epub 2022 Apr 2. Hepatol Commun. 2022. PMID: 35368148 Free PMC article.
-
Hepatitis B Gene Therapy Coming to Age.AIDS Rev. 2018 Apr-Jun;20(2):125-127. AIDS Rev. 2018. PMID: 29938706 Review.
Cited by
-
Recent Drug Development in the Woodchuck Model of Chronic Hepatitis B.Viruses. 2022 Aug 3;14(8):1711. doi: 10.3390/v14081711. Viruses. 2022. PMID: 36016334 Free PMC article. Review.
-
Rosmarinic acid is a novel inhibitor for Hepatitis B virus replication targeting viral epsilon RNA-polymerase interaction.PLoS One. 2018 May 21;13(5):e0197664. doi: 10.1371/journal.pone.0197664. eCollection 2018. PLoS One. 2018. PMID: 29782545 Free PMC article.
-
Classifying hepatitis B therapies with insights from covalently closed circular DNA dynamics.Virol Sin. 2024 Feb;39(1):9-23. doi: 10.1016/j.virs.2023.12.005. Epub 2023 Dec 16. Virol Sin. 2024. PMID: 38110037 Free PMC article. Review.
-
Editorial: In vitro mechanistic evaluation of nucleic acid polymers: A cautionary tale.Mol Ther Nucleic Acids. 2022 Mar 23;28:168-174. doi: 10.1016/j.omtn.2022.03.002. eCollection 2022 Jun 14. Mol Ther Nucleic Acids. 2022. PMID: 35402067 Free PMC article.
-
Nucleic acid polymer REP 2139 and nucleos(T)ide analogues act synergistically against chronic hepadnaviral infection in vivo in Pekin ducks.Hepatology. 2018 Jun;67(6):2127-2140. doi: 10.1002/hep.29737. Epub 2018 Feb 23. Hepatology. 2018. PMID: 29251788 Free PMC article.
References
-
- Bazinet M, Pantea V, Cebotarescu V, Cojuhari L, Jimbei P, Krawczyk A, Vaillant A. Safety and efficacy of REP 2139 and pegylated interferon alpha 2a in patients with chronic hepatitis B virus and hepatitis D virus coinfection: a phase 2 study. Lancet Gastroenterol Hepatol. 2017a;2017 (in press) - PubMed
-
- Bazinet M, Pantea V, Placinta G, Moscalu I, Cebotarescu V, Cojuhari L, Jimbei P, Iarovoi L2, Smesnoi V, Musteata T, Jucov A, Krawczyk A, Vaillant A. Update on safety and efficacy in the REP 401 protocol: REP 2139-Mg or REP 2165-Mg used in combination with tenofovir disoproxil fumarate and pegylated Interferon alpha-2a in treatment naïve caucasian patients with chronic HBeAg negative HBV infection. J Hepatol. 2017b;66:S256.
-
- Billioud G, Kurse RL, Carrillo M, Whitten-Bauer C, Gao D, Kim A, Chen L, McCaleb ML, Crosby JR, Hamatake R, Hong Z, Garaigorta U, Swazye E, Bisig KD, Wieland S. In vivo reduction of hepatitis B virus antigenemia and virema by antisense oligonucleotides. J Hepatol. 2016;64:781–789. - PubMed
-
- Blanchet M, Vaillant A, Labonte P. Post-entry antiviral effects of nucleic acid polymers against hepatitis B virus infection in vitro. J Hepatol. 2017;66:S257.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

