Quantitative Analysis of Kynurenine Aminotransferase II in the Adult Rat Brain Reveals High Expression in Proliferative Zones and Corpus Callosum
- PMID: 29126954
- PMCID: PMC5766363
- DOI: 10.1016/j.neuroscience.2017.11.001
Quantitative Analysis of Kynurenine Aminotransferase II in the Adult Rat Brain Reveals High Expression in Proliferative Zones and Corpus Callosum
Abstract
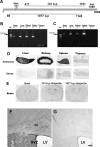
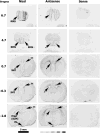
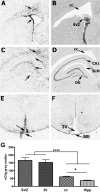
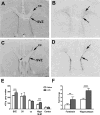
Kynurenic acid, a metabolite of the kynurenine pathway of tryptophan degradation, acts as an endogenous antagonist of alpha7 nicotinic and NMDA receptors and is implicated in a number of neurophysiological and neuropathological processes including cognition and neurodegenerative events. Therefore, kynurenine aminotransferase II (KAT II/AADAT), the enzyme responsible for the formation of the majority of neuroactive kynurenic acid in the brain, has prompted significant interest. Using immunohistochemistry, this enzyme was localized primarily in astrocytes throughout the adult rat brain, but detailed neuroanatomical studies are lacking. Here, we employed quantitative in situ hybridization to analyze the relative expression of KAT II mRNA in the brain of rats under normal conditions and 6 h after the administration of lipopolysaccharides (LPSs). Specific hybridization signals for KAT II were detected, with the highest expression in the subventricular zone (SVZ), the rostral migratory stream and the floor of the third ventricle followed by the corpus callosum and the hippocampus. This pattern of mRNA expression was paralleled by differential protein expression, determined by serial dilutions of antibodies (up to 1:1 million), and was confirmed to be primarily astrocytic in nature. The mRNA signal in the SVZ and the hippocampus was substantially increased by the LPS treatment without detectable changes elsewhere. These results demonstrate that KAT II is expressed in the rat brain in a region-specific manner and that gene expression is sensitive to inflammatory processes. This suggests an unrecognized role for kynurenic acid in the brain's germinal zones.
Keywords: astrocytes; doublecortin; in situ hybridization; lipopolysaccharides; subventricular zone; tanycytes.
Published by Elsevier Ltd.
Conflict of interest statement
All the authors declare no conflict of interest.
Figures





Similar articles
-
Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry.Glia. 2007 Jan 1;55(1):78-92. doi: 10.1002/glia.20432. Glia. 2007. PMID: 17024659
-
Astrocytic and neuronal localization of kynurenine aminotransferase-2 in the adult mouse brain.Brain Struct Funct. 2017 May;222(4):1663-1672. doi: 10.1007/s00429-016-1299-5. Epub 2016 Aug 27. Brain Struct Funct. 2017. PMID: 27568378
-
Ammonia upregulates kynurenine aminotransferase II mRNA expression in rat brain: a role for astrocytic NMDA receptors?Metab Brain Dis. 2013 Jun;28(2):161-5. doi: 10.1007/s11011-012-9353-3. Epub 2012 Nov 8. Metab Brain Dis. 2013. PMID: 23132651
-
The kynurenine pathway in schizophrenia and bipolar disorder.Neuropharmacology. 2017 Jan;112(Pt B):297-306. doi: 10.1016/j.neuropharm.2016.05.020. Epub 2016 May 28. Neuropharmacology. 2017. PMID: 27245499 Review.
-
Kynurenic acid and kynurenine aminotransferases in retinal aging and neurodegeneration.Pharmacol Rep. 2011;63(6):1324-34. doi: 10.1016/s1734-1140(11)70697-1. Pharmacol Rep. 2011. PMID: 22358081 Review.
Cited by
-
Hippocampal regenerative medicine: neurogenic implications for addiction and mental disorders.Exp Mol Med. 2021 Mar;53(3):358-368. doi: 10.1038/s12276-021-00587-x. Epub 2021 Mar 30. Exp Mol Med. 2021. PMID: 33785869 Free PMC article. Review.
-
Angiotensin II type 1 receptor blockers decrease kynurenic acid production in rat kidney in vitro.Naunyn Schmiedebergs Arch Pharmacol. 2019 Feb;392(2):209-217. doi: 10.1007/s00210-018-1572-7. Epub 2018 Oct 29. Naunyn Schmiedebergs Arch Pharmacol. 2019. PMID: 30370429
-
Association of complement component 4 with neuroimmune abnormalities in the subventricular zone in schizophrenia and autism spectrum disorders.Neurobiol Dis. 2022 Oct 15;173:105840. doi: 10.1016/j.nbd.2022.105840. Epub 2022 Aug 19. Neurobiol Dis. 2022. PMID: 35995342 Free PMC article.
-
The Complex World of Kynurenic Acid: Reflections on Biological Issues and Therapeutic Strategy.Int J Mol Sci. 2024 Aug 20;25(16):9040. doi: 10.3390/ijms25169040. Int J Mol Sci. 2024. PMID: 39201726 Free PMC article. Review.
-
scEWE: high-order element-wise weighted ensemble clustering for heterogeneity analysis of single-cell RNA-sequencing data.Brief Bioinform. 2024 Mar 27;25(3):bbae203. doi: 10.1093/bib/bbae203. Brief Bioinform. 2024. PMID: 38701413 Free PMC article.
References
-
- Balazs R. Trophic effect of glutamate. Curr Top Med Chem. 2006;6:961–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

