The high-production volume fungicide pyraclostrobin induces triglyceride accumulation associated with mitochondrial dysfunction, and promotes adipocyte differentiation independent of PPARγ activation, in 3T3-L1 cells
- PMID: 29127035
- PMCID: PMC5726929
- DOI: 10.1016/j.tox.2017.11.010
The high-production volume fungicide pyraclostrobin induces triglyceride accumulation associated with mitochondrial dysfunction, and promotes adipocyte differentiation independent of PPARγ activation, in 3T3-L1 cells
Abstract
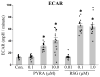
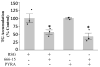
Pyraclostrobin is one of the most heavily used fungicides, and has been detected on a variety of produce, suggesting human exposure occurs regularly. Recently, pyraclostrobin exposure has been linked to a variety of toxic effects, including neurodegeneration and triglyceride (TG) accumulation. As pyraclostrobin inhibits electron transport chain complex III, and as mitochondrial dysfunction is associated with metabolic syndrome (cardiovascular disease, type II diabetes, obesity), we designed experiments to test the hypothesis that mitochondrial dysfunction underlies its adipogenic activity. 3T3-L1 cells were differentiated according to standard protocols in the presence of pyraclostrobin, resulting in TG accumulation. However, TG accumulation occurred without activation of the peroxisome proliferator activated nuclear receptor gamma (PPARγ), the canonical pathway mediating adipogenesis. Furthermore, cells failed to express many markers of adipogenesis (PPARγ, lpl, CEBPα), while co-exposure to pyraclostrobin and two different PPARγ antagonists (GW9662, T0070907) failed to mitigate TG accumulation, suggesting TG accumulation occurred through a PPARγ-independent mechanism. Instead, pyraclostrobin reduced steady-state ATP, mitochondrial membrane potential, basal mitochondrial respiration, ATP-linked respiration, and spare respiratory capacity, demonstrating mitochondrial dysfunction, while reduced expression of genes involved in glucose transport (Glut-4), glycolysis (Pkm, Pfkl, Pfkm), fatty acid oxidation (Cpt-1b), and lipogenesis (Fasn, Acacα, Acacβ) further suggested a disruption of metabolism. Finally, inhibition of cAMP responsive element binding protein (CREB), a PPARγ coactivator, partially mitigated pyraclostrobin-induced TG accumulation, suggesting TG accumulation is occurring through a CREB-driven mechanism. In contrast, rosiglitazone, a known PPARγ agonist, induced TG accumulation in a PPARγ-dependent manner and enhanced mitochondrial function. Collectively, these results suggest pyraclostrobin-induced mitochondrial dysfunction inhibits lipid homeostasis, resulting in TG accumulation. Exposures that disrupt mitochondrial function may have the potential to contribute to the rising incidence of metabolic syndrome, and thus more research is needed to understand the human health impact of pyraclostrobin exposure.
Keywords: Adipogenesis; Fungicide; Metabolic disruption; Mitochondrial toxicity; Pyraclostrobin.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures

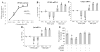
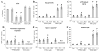
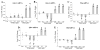

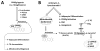
Similar articles
-
Suppression of adipocyte differentiation and lipid accumulation by stearidonic acid (SDA) in 3T3-L1 cells.Lipids Health Dis. 2017 Sep 25;16(1):181. doi: 10.1186/s12944-017-0574-7. Lipids Health Dis. 2017. PMID: 28946872 Free PMC article.
-
Mitochondrial dysfunction induces triglyceride accumulation in 3T3-L1 cells: role of fatty acid beta-oxidation and glucose.J Lipid Res. 2005 Jun;46(6):1133-49. doi: 10.1194/jlr.M400464-JLR200. Epub 2005 Mar 1. J Lipid Res. 2005. PMID: 15741651
-
Obestatin and Rosiglitazone Differentially Modulate Lipid Metabolism Through Peroxisome Proliferator-activated Receptor-γ (PPARγ) in Pre-adipose and Mature 3T3-L1 Cells.Cell Biochem Biophys. 2021 Mar;79(1):73-85. doi: 10.1007/s12013-020-00958-7. Epub 2021 Jan 11. Cell Biochem Biophys. 2021. PMID: 33432549
-
A comprehensive review of strobilurin fungicide toxicity in aquatic species: Emphasis on mode of action from the zebrafish model.Environ Pollut. 2021 Apr 15;275:116671. doi: 10.1016/j.envpol.2021.116671. Epub 2021 Feb 6. Environ Pollut. 2021. PMID: 33582629 Review.
-
Toxicological impact of strobilurin fungicides on human and environmental health: a literature review.J Environ Sci Health B. 2024;59(4):142-151. doi: 10.1080/03601234.2024.2312786. Epub 2024 Feb 11. J Environ Sci Health B. 2024. PMID: 38343082 Review.
Cited by
-
Reproducibility of adipogenic responses to metabolism disrupting chemicals in the 3T3-L1 pre-adipocyte model system: An interlaboratory study.Toxicology. 2021 Sep;461:152900. doi: 10.1016/j.tox.2021.152900. Epub 2021 Aug 17. Toxicology. 2021. PMID: 34411659 Free PMC article.
-
Mitochondrial and Endoplasmic Reticulum Stress Trigger Triglyceride Accumulation in Models of Parkinson's Disease Independent of Mutations in MAPT.Metabolites. 2023 Jan 9;13(1):112. doi: 10.3390/metabo13010112. Metabolites. 2023. PMID: 36677037 Free PMC article.
-
Alternative polyadenylation landscape of longissimus dorsi muscle with high and low intramuscular fat content in cattle.J Anim Sci. 2024 Jan 3;102:skae357. doi: 10.1093/jas/skae357. J Anim Sci. 2024. PMID: 39565284
-
cAMP Response Element Binding Protein 1 (CREB1) Promotes Monounsaturated Fatty Acid Synthesis and Triacylglycerol Accumulation in Goat Mammary Epithelial Cells.Animals (Basel). 2020 Oct 14;10(10):1871. doi: 10.3390/ani10101871. Animals (Basel). 2020. PMID: 33066354 Free PMC article.
-
Quizalofop-p-Ethyl Induces Adipogenesis in 3T3-L1 Adipocytes.Toxicol Sci. 2019 Aug 1;170(2):452-461. doi: 10.1093/toxsci/kfz097. Toxicol Sci. 2019. PMID: 31086981 Free PMC article.
References
-
- Amuna P, Zotor FB. Epidemiological and nutrition transition in developing countries: impact on human health and development. Proceedings of the Nutrition Society. 2008;67(01):82–90. - PubMed
-
- Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Molecular cell. 1999;4(4):585–95. - PubMed
-
- Battaglin WA, Sandstrom MW, Kuivila KM, Kolpin DW, Meyer MT. Occurrence of azoxystrobin, propiconazole, and selected other fungicides in US streams, 2005–2006. Water, Air, & Soil Pollution. 2011;218(1–4):307–322.
-
- Becker W, Von Jagow G, Anke T, Steglich W. Oudemansin, strobilurin A, strobilurin B and myxothiazol: new inhibitors of the bc1 segment of the respiratory chain with an E-β-methoxyacrylate system as common structural element. FEBS letters. 1981;132(2):329–333. - PubMed
-
- Chandel NS. Navigating metabolism. Cold Spring Harbor Laboratory; 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

