The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration
- PMID: 29127045
- PMCID: PMC5723221
- DOI: 10.1016/j.semcdb.2017.11.010
The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration
Abstract
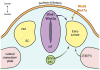
The Myogenic Regulatory Factors (MRFs) Myf5, MyoD, myogenin and MRF4 are members of the basic helix-loop-helix family of transcription factors that control the determination and differentiation of skeletal muscle cells during embryogenesis and postnatal myogenesis. The dynamics of their temporal and spatial expression as well as their biochemical properties have allowed the identification of a precise and hierarchical relationship between the four MRFs. This relationship establishes the myogenic lineage as well as the maintenance of the terminal myogenic phenotype. The application of genome-wide technologies has provided important new information as to how the MRFs function to activate muscle gene expression. Application of combined functional genomics technologies along with single cell lineage tracing strategies will allow a deeper understanding of the mechanisms mediating myogenic determination, cell differentiation and muscle regeneration.
Keywords: MyoD; Myogenesis; Myogenic regulatory factors; Skeletal muscle.
Copyright © 2017 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

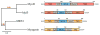
References
-
- Buckingham M, Rigby PWJ. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev Cell. 2014 Feb 10;28(3):225–38. - PubMed
-
- Lassar AB, Paterson BM, Weintraub H. Transfection of a DNA locus that mediates the conversion of 10T1/2 fibroblasts to myoblasts. Cell. 1986 Dec 5;47(5):649–56. - PubMed
-
- Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra R, et al. The myod gene family: Nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–6. - PubMed
-
- Lassar AB, Davis RL, Wright WE, Kadesch T, Murre C, Voronova A, et al. Functional activity of myogenic HLH proteins requires hetero-oligomerization with E12/e47-like proteins in vivo. Cell. 1991 Jul 26;66(2):305–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

