Granulocyte colony-stimulating factor and autologous CD133-positive stem-cell therapy in liver cirrhosis (REALISTIC): an open-label, randomised, controlled phase 2 trial
- PMID: 29127060
- PMCID: PMC5738975
- DOI: 10.1016/S2468-1253(17)30326-6
Granulocyte colony-stimulating factor and autologous CD133-positive stem-cell therapy in liver cirrhosis (REALISTIC): an open-label, randomised, controlled phase 2 trial
Abstract
Background: Results of small-scale studies have suggested that stem-cell therapy is safe and effective in patients with liver cirrhosis, but no adequately powered randomised controlled trials have been done. We assessed the safety and efficacy of granulocyte colony-stimulating factor (G-CSF) and haemopoietic stem-cell infusions in patients with liver cirrhosis.
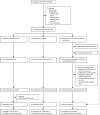
Methods: This multicentre, open-label, randomised, controlled phase 2 trial was done in three UK hospitals and recruited patients with compensated liver cirrhosis and MELD scores of 11·0-15·5. Patients were randomly assigned (1:1:1) to receive standard care (control), treatment with subcutaneous G-CSF (lenograstim) 15 μg/kg for 5 days, or treatment with G-CSF for 5 days followed by leukapheresis and intravenous infusion of three doses of CD133-positive haemopoietic stem cells (0·2 × 106 cells per kg per infusion). Randomisation was done by Cancer Research UK Clinical Trials Unit staff with a minimisation algorithm that stratified by trial site and cause of liver disease. The coprimary outcomes were improvement in severity of liver disease (change in MELD) at 3 months and the trend of change in MELD score over time. Analyses were done in the modified intention-to-treat population, which included all patients who received at least one day of treatment. Safety was assessed on the basis of the treatment received. This trial was registered at Current Controlled Trials on Nov 18, 2009; ISRCTN, number 91288089; and the European Clinical Trials Database, number 2009-010335-41.
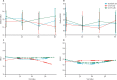
Findings: Between May 18, 2010, and Feb 26, 2015, 27 patients were randomly assigned to the standard care, 26 to the G-CSF group, and 28 to the G-CSF plus stem-cell infusion group. Median change in MELD from day 0 to 90 was -0·5 (IQR -1·5 to 1·1) in the standard care group, -0·5 (-1·7 to 0·5) in the G-CSF group, and -0·5 (-1·3 to 1·0) in the G-CSF plus stem-cell infusion group. We found no evidence of differences between the treatment groups and control group in the trends of MELD change over time (p=0·55 for the G-CSF group vs standard care and p=0·75 for the G-CSF plus stem-cell infusion group vs standard care). Serious adverse events were more frequent the in G-CSF and stem-cell infusion group (12 [43%] patients) than in the G-CSF (three [11%] patients) and standard care (three [12%] patients) groups. The most common serious adverse events were ascites (two patients in the G-CSF group and two patients in the G-CSF plus stem-cell infusion group, one of whom was admitted to hospital with ascites twice), sepsis (four patients in the G-CSF plus stem-cell infusion group), and encephalopathy (three patients in the G-CSF plus stem-cell infusion group, one of whom was admitted to hospital with encephalopathy twice). Three patients died, including one in the standard care group (variceal bleed) and two in the G-CSF and stem-cell infusion group (one myocardial infarction and one progressive liver disease).
Interpretation: G-CSF with or without haemopoietic stem-cell infusion did not improve liver dysfunction or fibrosis and might be associated with increased frequency of adverse events compared with standard care.
Funding: National Institute of Health Research, The Sir Jules Thorn Charitable Trust.
Copyright © 2018 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Haemopoietic stem cell therapy in cirrhosis: the end of the story?Lancet Gastroenterol Hepatol. 2018 Jan;3(1):3-5. doi: 10.1016/S2468-1253(17)30359-X. Epub 2017 Nov 7. Lancet Gastroenterol Hepatol. 2018. PMID: 29127062 No abstract available.
-
Stem cells: Stem cell therapy for liver cirrhosis unREALISTIC?Nat Rev Gastroenterol Hepatol. 2018 Jan;15(1):4. doi: 10.1038/nrgastro.2017.178. Epub 2017 Dec 13. Nat Rev Gastroenterol Hepatol. 2018. PMID: 29235547 No abstract available.
-
Haematopoietic stem cells in cirrhosis - Authors' reply.Lancet Gastroenterol Hepatol. 2018 May;3(5):298-299. doi: 10.1016/S2468-1253(18)30035-9. Epub 2018 Apr 6. Lancet Gastroenterol Hepatol. 2018. PMID: 29644971 No abstract available.
-
Haematopoietic stem cells in cirrhosis.Lancet Gastroenterol Hepatol. 2018 May;3(5):298. doi: 10.1016/S2468-1253(18)30041-4. Epub 2018 Apr 6. Lancet Gastroenterol Hepatol. 2018. PMID: 29644972 No abstract available.
Similar articles
-
REpeated AutoLogous Infusions of STem cells In Cirrhosis (REALISTIC): a multicentre, phase II, open-label, randomised controlled trial of repeated autologous infusions of granulocyte colony-stimulating factor (GCSF) mobilised CD133+ bone marrow stem cells in patients with cirrhosis. A study protocol for a randomised controlled trial.BMJ Open. 2015 Mar 20;5(3):e007700. doi: 10.1136/bmjopen-2015-007700. BMJ Open. 2015. PMID: 25795699 Free PMC article. Clinical Trial.
-
Safety and efficacy of autologous haematopoietic stem-cell transplantation with low-dose cyclophosphamide mobilisation and reduced intensity conditioning versus standard of care in refractory Crohn's disease (ASTIClite): an open-label, multicentre, randomised controlled trial.Lancet Gastroenterol Hepatol. 2024 Apr;9(4):333-345. doi: 10.1016/S2468-1253(23)00460-0. Epub 2024 Feb 7. Lancet Gastroenterol Hepatol. 2024. PMID: 38340759 Clinical Trial.
-
Multiple cycles of granulocyte colony-stimulating factor in decompensated cirrhosis: a double-blind RCT.Hepatol Int. 2022 Oct;16(5):1127-1136. doi: 10.1007/s12072-022-10314-x. Epub 2022 Mar 23. Hepatol Int. 2022. PMID: 35322373 Free PMC article. Clinical Trial.
-
The effect of granulocyte-colony stimulating factor, decitabine, and busulfan-cyclophosphamide versus busulfan-cyclophosphamide conditioning on relapse in patients with myelodysplastic syndrome or secondary acute myeloid leukaemia evolving from myelodysplastic syndrome undergoing allogeneic haematopoietic stem-cell transplantation: an open-label, multicentre, randomised, phase 3 trial.Lancet Haematol. 2023 Mar;10(3):e178-e190. doi: 10.1016/S2352-3026(22)00375-1. Epub 2023 Jan 23. Lancet Haematol. 2023. PMID: 36702138 Clinical Trial.
-
Granulocyte-Colony Stimulating Factor (G-CSF) for stroke: an individual patient data meta-analysis.Sci Rep. 2016 Nov 15;6:36567. doi: 10.1038/srep36567. Sci Rep. 2016. PMID: 27845349 Free PMC article. Review.
Cited by
-
Clinical application of stem cell in patients with end-stage liver disease: progress and challenges.Ann Transl Med. 2020 Apr;8(8):564. doi: 10.21037/atm.2020.03.153. Ann Transl Med. 2020. PMID: 32775365 Free PMC article. Review.
-
Antimicrobial resistance in chronic liver disease.Hepatol Int. 2020 Jan;14(1):24-34. doi: 10.1007/s12072-019-10004-1. Epub 2019 Dec 3. Hepatol Int. 2020. PMID: 31797303 Free PMC article. Review.
-
Causes and Consequences of Innate Immune Dysfunction in Cirrhosis.Front Immunol. 2019 Feb 25;10:293. doi: 10.3389/fimmu.2019.00293. eCollection 2019. Front Immunol. 2019. PMID: 30873165 Free PMC article. Review.
-
Role of the Gut-Liver Axis in Liver Inflammation, Fibrosis, and Cancer: A Special Focus on the Gut Microbiota Relationship.Hepatol Commun. 2019 Mar 1;3(4):456-470. doi: 10.1002/hep4.1331. eCollection 2019 Apr. Hepatol Commun. 2019. PMID: 30976737 Free PMC article. Review.
-
A first-in-human clinical study of laparoscopic autologous myoblast sheet transplantation to prevent delayed perforation after duodenal endoscopic mucosal dissection.Stem Cell Res Ther. 2024 Apr 23;15(1):117. doi: 10.1186/s13287-024-03730-3. Stem Cell Res Ther. 2024. PMID: 38654373 Free PMC article.
References
-
- Williams R, Aspinall R, Bellis M. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet. 2014;384:1953–1997. - PubMed
-
- Di Marco V, Calvaruso V, Ferraro D. Effects of eradicating hepatitis C virus infection in patients with cirrhosis differ with stage of portal hypertension. Gastroenterology. 2016;151:130–139. e2. - PubMed
-
- Thomas JA, Pope C, Wojtacha D. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration and function. Hepatology. 2011;53:2003–2015. - PubMed
-
- Sakaida I, Terai S, Yamamoto N. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304–1311. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

