A Naturalistic, Randomized Pilot Trial of E-Cigarettes: Uptake, Exposure, and Behavioral Effects
- PMID: 29127080
- PMCID: PMC5713898
- DOI: 10.1158/1055-9965.EPI-17-0460
A Naturalistic, Randomized Pilot Trial of E-Cigarettes: Uptake, Exposure, and Behavioral Effects
Abstract
Background: Most studies of electronic nicotine delivery systems (ENDS) compare self-selected users versus nonusers. The few randomized studies to date generally support a positive impact on reducing smoking behavior, but these studies are focused on guided ENDS use. This study presents a randomized, naturalistic trial of ENDS with prospective outcomes of uptake and behavioral changes in smoking.Methods: Adult smokers with minimal ENDS history were randomized in a 2:1 ratio to receive product for 3 weeks (n = 46), or not (n = 22). Changes in nicotine delivery (16 vs. 24 mg), midway through the study allowed a compelling opportunity to examine two ENDS products compared with the control group. Primary outcomes, assessed via daily diaries during sampling period and in-person laboratory visits over 4 months, included uptake and usage of ENDS, cessation-related outcomes, and exposure to smoke constituents.Results: All ENDS participants tried product at least once, with 48% of 24 mg and 30% of 16 mg using their assigned product for the entire sampling period. Within the 24 mg ENDs group, 57% made an independent purchase of ENDS, versus 28% of 16 mg, and 14% of control participants (P = 0.01). Smokers in both ENDS groups significantly reduced their smoking, whereas control participants did not (P = 0.03). Cessation behaviors (quit attempts, biologically verified abstinence) numerically but not statistically favored ENDS participants.Conclusions: Results suggest that cigarette smokers are willing to use ENDS with trends toward reduced cigarette smoking and positive changes in cessation-related behaviors.Impact: Randomized, naturalistic trials such as presented herein are needed to understand the population impact of e-cigarettes. Cancer Epidemiol Biomarkers Prev; 26(12); 1795-803. ©2017 AACR.
©2017 American Association for Cancer Research.
Figures
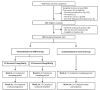
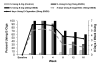

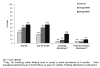
References
-
- McRobbie H, Bullen C, Hartmann-Boyce J, Hajek P. Electronic cigarettes for smoking cessation and reduction Cochrane Database of Systematice Reviews. Oxford: John Wiley & Sons; 2014. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

