Therapeutic potential of microRNAs for the treatment of renal fibrosis and CKD
- PMID: 29127220
- PMCID: PMC5866411
- DOI: 10.1152/physiolgenomics.00039.2017
Therapeutic potential of microRNAs for the treatment of renal fibrosis and CKD
Abstract
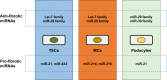
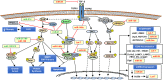
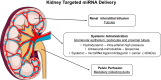
Chronic kidney disease (CKD), defined as reduced glomerular filtration rate, is increasingly becoming a major public health issue. At the histological level, renal fibrosis is the final common pathway leading to end-stage renal disease, irrespective of the initial injury. According to this view, antifibrotic agents should slow or halt the progression of CKD. However, due to multiple overlapping pathways stimulating fibrosis, it has been difficult to develop antifibrotic drugs that delay or reverse the progression of CKD. MicroRNAs (miRNAs) are small noncoding RNA molecules, 18-22 nucleotides in length, that control many developmental and cellular processes as posttranscriptional regulators of gene expression. Emerging evidence suggests that miRNAs targeted against genes involved in renal fibrosis might be potential candidates for the development of antifibrotic therapies for CKD. This review will discuss some of the miRNAs, such as Let-7, miR-21,-29, -192, -200,-324, -132, -212, -30, -126, -433, -214, and -199a, that are implicated in renal fibrosis and the potential to exploit these molecular targets for the treatment of CKD.
Keywords: chronic kidney disease; microRNA; renal fibrosis.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

