Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice
- PMID: 29127332
- PMCID: PMC5681558
- DOI: 10.1038/s41467-017-01484-6
Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice
Abstract
Cerebrospinal fluid (CSF) has been commonly accepted to drain through arachnoid projections from the subarachnoid space to the dural venous sinuses. However, a lymphatic component to CSF outflow has long been known. Here, we utilize lymphatic-reporter mice and high-resolution stereomicroscopy to characterize the anatomical routes and dynamics of outflow of CSF. After infusion into a lateral ventricle, tracers spread into the paravascular spaces of the pia mater and cortex of the brain. Tracers also rapidly reach lymph nodes using perineural routes through foramina in the skull. Using noninvasive imaging techniques that can quantify the transport of tracers to the blood and lymph nodes, we find that lymphatic vessels are the major outflow pathway for both large and small molecular tracers in mice. A significant decline in CSF lymphatic outflow is found in aged compared to young mice, suggesting that the lymphatic system may represent a target for age-associated neurological conditions.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

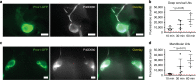
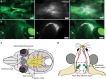
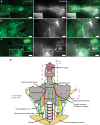
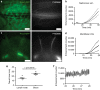
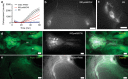
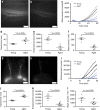
References
-
- Davson, H. & Segal, M. B. Physiology of the CSF and Blood-Brain Barriers (CRC, Boca Raton, FL, 1996).
-
- Cserr, H. F. in Hydrocephalus (eds Shapiro, K., Marmarou, A. & Portnoy, H.) 59–68 (Raven, New York, NY, 1984).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

