Catabolic Effects of Human PTH (1-34) on Bone: Requirement of Monocyte Chemoattractant Protein-1 in Murine Model of Hyperparathyroidism
- PMID: 29127344
- PMCID: PMC5681546
- DOI: 10.1038/s41598-017-15563-7
Catabolic Effects of Human PTH (1-34) on Bone: Requirement of Monocyte Chemoattractant Protein-1 in Murine Model of Hyperparathyroidism
Abstract
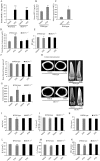
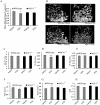
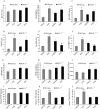
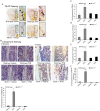
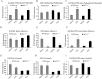
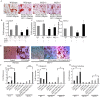
The bone catabolic actions of parathyroid hormone (PTH) are seen in patients with hyperparathyroidism, or with infusion of PTH in rodents. We have previously shown that the chemokine, monocyte chemoattractant protein-1 (MCP-1), is a mediator of PTH's anabolic effects on bone. To determine its role in PTH's catabolic effects, we continuously infused female wild-type (WT) and MCP-1-/- mice with hPTH or vehicle. Microcomputed tomography (µCT) analysis of cortical bone showed that hPTH-infusion induced significant bone loss in WT mice. Further, μCT analysis of trabecular bone revealed that, compared with the vehicle-treated group, the PTH-treated WT mice had reduced trabecular thickness and trabecular number. Notably, MCP-1-/- mice were protected against PTH-induced cortical and trabecular bone loss as well as from increases in serum CTX (C-terminal crosslinking telopeptide of type I collagen) and TRACP-5b (tartrate-resistant acid phosphatase 5b). In vitro, bone marrow macrophages (BMMs) from MCP-1-/- and WT mice were cultured with M-CSF, RANKL and/or MCP-1. BMMs from MCP-1-/- mice showed decreased multinucleated osteoclast formation compared with WT mice. Taken together, our work demonstrates that MCP-1 has a role in PTH's catabolic effects on bone including monocyte and macrophage recruitment, osteoclast formation, bone resorption, and cortical and trabecular bone loss.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Nakayama K, et al. Differences in bone and vitamin D metabolism between primary hyperparathyroidism and malignancy-associated hypercalcemia. J. Clin. Endocrinol. Metab. 1996;81:607–611. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

