Suppression of Th17-polarized airway inflammation by rapamycin
- PMID: 29127369
- PMCID: PMC5681547
- DOI: 10.1038/s41598-017-15750-6
Suppression of Th17-polarized airway inflammation by rapamycin
Abstract
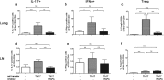
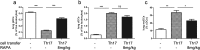
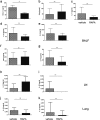
Because Th17-polarized airway inflammation correlates with poor control in bronchial asthma and is a feature of numerous other difficult-to-treat inflammatory lung diseases, new therapeutic approaches for this type of airway inflammation are necessary. We assessed different licensed anti-inflammatory agents with known or expected efficacy against Th17-polarization in mouse models of Th17-dependent airway inflammation. Upon intravenous transfer of in vitro derived Th17 cells and intranasal challenge with the corresponding antigen, we established acute and chronic murine models of Th17-polarised airway inflammation. Consecutively, we assessed the efficacy of methylprednisolone, roflumilast, azithromycin, AM80 and rapamycin against acute or chronic Th17-dependent airway inflammation. Quantifiers for Th17-associated inflammation comprised: bronchoalveolar lavage (BAL) differential cell counts, allergen-specific cytokine and immunoglobulin secretion, as well as flow cytometric phenotyping of pulmonary inflammatory cells. Only rapamycin proved effective against acute Th17-dependent airway inflammation, accompanied by increased plasmacytoid dendritic cells (pDCs) and reduced neutrophils as well as reduced CXCL-1 levels in BAL. Chronic Th17-dependent airway inflammation was unaltered by rapamycin treatment. None of the other agents showed efficacy in our models. Our results demonstrate that Th17-dependent airway inflammation is difficult to treat with known agents. However, we identify rapamycin as an agent with inhibitory potential against acute Th17-polarized airway inflammation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

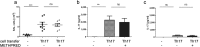


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

