Performance of Solid-state Hybrid Energy-storage Device using Reduced Graphene-oxide Anchored Sol-gel Derived Ni/NiO Nanocomposite
- PMID: 29127411
- PMCID: PMC5681587
- DOI: 10.1038/s41598-017-15444-z
Performance of Solid-state Hybrid Energy-storage Device using Reduced Graphene-oxide Anchored Sol-gel Derived Ni/NiO Nanocomposite
Abstract
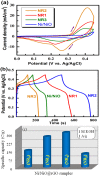
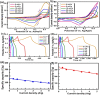
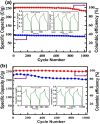
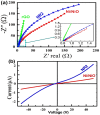
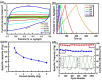
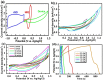
The influence of (nickel nitrate/citric acid) mole ratio on the formation of sol-gel end products was examined. The formed Ni/NiO nanoparticle was anchored on to reduced graphene-oxide (rGO) by means of probe sonication. It was found that the sample obtained from the (1:1) nickel ion: citric acid (Ni2+: CA) mole ratio resulted in a high specific capacity of 158 C/g among all (Ni2+: CA) ratios examined. By anchoring Ni/NiO on to the rGO resulted in enhanced specific capacity of as high as 335 C/g along with improved cycling stability, high rate capability and Coulombic efficiency. The high conductivity and increased surface area seemed responsible for enhanced electrochemical performances of the Ni/NiO@rGO nanocomposite. A solid-state hybrid energy-storage device consisting of the Ni/NiO@rGO (NR2) as a positive electrode and the rGO as negative electrode exhibited enhanced energy and power densities. Lighting of LED was demonstrated by using three proto-type (NR2(+)|| rGO(-)) hybrid devices connected in series.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Lokhande CD, Dubal DP, Joo O-S. Metal oxide thin film based supercapacitors. Curr. Appl. Phys. 2011;11:255–270. doi: 10.1016/j.cap.2010.12.001. - DOI
-
- Li Z, et al. Improved synthesis of fluffy and wrinkled reduced graphene oxide for energy storage application. Vacuum. 2015;117:35–39. doi: 10.1016/j.vacuum.2015.03.032. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

