Hearing with exceptionally thin tympana: Ear morphology and tympanal membrane vibrations in eneopterine crickets
- PMID: 29127426
- PMCID: PMC5681576
- DOI: 10.1038/s41598-017-15282-z
Hearing with exceptionally thin tympana: Ear morphology and tympanal membrane vibrations in eneopterine crickets
Abstract
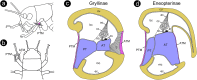
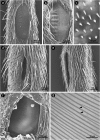
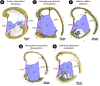
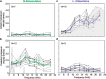
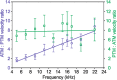
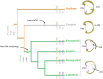
The receiver sensory system plays a crucial role in the evolution of new communication signals in insects. Among acoustic communicating crickets, the tribe Lebinthini (Eneopterinae) has evolved a unique communication system in that males produce exceptionally high-frequency calls and females respond with vibratory signals to guide males towards them. In this study, we describe nine species of Eneopterinae in which the sound receiving structures have undergone considerable morphological changes. We revealed that the anterior tympanal membrane (ATM) of the ear was extremely thin, as little as 0.35 µm thick, and to the best of our knowledge, this is the thinnest tympanal membrane found in crickets thus far. Measurements of tympanum vibrations obtained from Lebinthus bitaeniatus demonstrated a strong sensitivity towards higher frequencies. The finding also coincides with the neuronal tuning of ascending neurons and the behavioural response of the Lebinthini. The morphologically specialized ATM and its mechanical sensitivity for high frequencies, therefore, may have driven the sensory exploitation of an anti-predator behaviour that led to the evolution of a new communication system known for this group of crickets. The hypothetical phylogenetic origin of the investigated tympanal ears is discussed.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Greenfield, M. D. Evolution of acoustic communication in insects in InsectHearing (eds. Pollack, G. S. et al.) 17–47 (Springer, 2016).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

