Amniotic membrane promotes focal adhesion remodeling to stimulate cell migration
- PMID: 29127427
- PMCID: PMC5681678
- DOI: 10.1038/s41598-017-15509-z
Amniotic membrane promotes focal adhesion remodeling to stimulate cell migration
Abstract
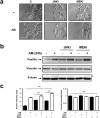
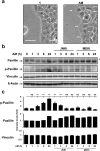
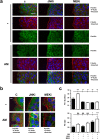
During wound healing, the migration of keratinocytes onto newly restored extracellular matrix aims to reestablish continuity of the epidermis. The application of amniotic membrane (AM) to chronic, deep traumatic, non-healing wounds has proven successful at stimulating re-epithelialization. When applied on epithelial cell cultures, AM activates extracellular signal-regulated kinases 1/2 (ERK1/2) and c-Jun N-terminal kinases 1/2 (JNK1/2), with the overexpression and phosphorylation of c-Jun along the wound edge. The effect of AM on the migration of cells was investigated by studying critical proteins involved in the focal adhesions turn-over: Focal Adhesion Kinase (FAK), Paxillin and Vinculin. In Mv1Lu and HaCaT cells, validated models for cell migration and wound healing, AM affected the expression and activation of Paxillin, but did not affect Vinculin expression, both factors which integrate into focal adhesions. Moreover, AM regulation also affected FAK activity through phosphorylation. Finally, we have determined that AM regulation of focal adhesions involves both JNK and MEK MAP kinase signaling pathways. This data provides a molecular background to understand how AM regulates critical cell and molecular aspects of cell migration, organizing and directing the movement of cells by the continuous formation, maturation, and turnover of focal adhesion structures at the migration leading edge.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Chronic Wound Healing by Amniotic Membrane: TGF-β and EGF Signaling Modulation in Re-epithelialization.Front Bioeng Biotechnol. 2021 Jul 6;9:689328. doi: 10.3389/fbioe.2021.689328. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34295882 Free PMC article. Review.
-
Role of formation of an ERK-FAK-paxillin complex in migration of human corneal epithelial cells during wound closure in vitro.Invest Ophthalmol Vis Sci. 2009 Dec;50(12):5646-52. doi: 10.1167/iovs.08-2534. Epub 2009 Jun 3. Invest Ophthalmol Vis Sci. 2009. PMID: 19494198
-
Cardiac fibroblasts require focal adhesion kinase for normal proliferation and migration.Am J Physiol Heart Circ Physiol. 2009 Mar;296(3):H627-38. doi: 10.1152/ajpheart.00444.2008. Epub 2009 Jan 9. Am J Physiol Heart Circ Physiol. 2009. PMID: 19136609 Free PMC article.
-
Phospho-regulated tethering of focal adhesion kinase to vinculin links force transduction to focal adhesion signaling.Cell Commun Signal. 2025 Apr 21;23(1):190. doi: 10.1186/s12964-025-02201-3. Cell Commun Signal. 2025. PMID: 40259376 Free PMC article.
-
Protein kinase C, focal adhesions and the regulation of cell migration.J Histochem Cytochem. 2014 Mar;62(3):172-84. doi: 10.1369/0022155413517701. Epub 2013 Dec 5. J Histochem Cytochem. 2014. PMID: 24309511 Free PMC article. Review.
Cited by
-
Application of Fetal Membranes and Natural Materials for Wound and Tissue Repair.Int J Mol Sci. 2024 Nov 5;25(22):11893. doi: 10.3390/ijms252211893. Int J Mol Sci. 2024. PMID: 39595963 Free PMC article. Review.
-
The Categorization of Perinatal Derivatives for Orthopedic Applications.Biomedicines. 2024 Jul 11;12(7):1544. doi: 10.3390/biomedicines12071544. Biomedicines. 2024. PMID: 39062117 Free PMC article. Review.
-
Comparison of the effects of preservation methods on structural, biological, and mechanical properties of the human amniotic membrane for medical applications.Cell Tissue Bank. 2024 Mar;25(1):305-323. doi: 10.1007/s10561-023-10114-z. Epub 2023 Oct 15. Cell Tissue Bank. 2024. PMID: 37840108 Review.
-
Tumor Targeting by Conditioned Medium Derived From Human Amniotic Membrane: New Insight in Breast Cancer Therapy.Technol Cancer Res Treat. 2021 Jan-Dec;20:15330338211036318. doi: 10.1177/15330338211036318. Technol Cancer Res Treat. 2021. PMID: 34402329 Free PMC article.
-
Chronic Wound Healing by Amniotic Membrane: TGF-β and EGF Signaling Modulation in Re-epithelialization.Front Bioeng Biotechnol. 2021 Jul 6;9:689328. doi: 10.3389/fbioe.2021.689328. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34295882 Free PMC article. Review.
References
-
- Barrientos, S., Stojadinovic, O., Golinko, M. S., Brem, H. & Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society 16, 585–601, 10.1111/j.1524-475X.2008.00410.x (2008). - PubMed
-
- Castellanos, G., Bernabe-Garcia, A., Moraleda, J. M. & Nicolas, F. J. Amniotic membrane application for the healing of chronic wounds and ulcers. Placenta, 10.1016/j.placenta.2017.04.005 (2017). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

