International development of four EORTC disease-specific quality of life questionnaires for patients with Hodgkin lymphoma, high- and low-grade non-Hodgkin lymphoma and chronic lymphocytic leukaemia
- PMID: 29127596
- PMCID: PMC5846994
- DOI: 10.1007/s11136-017-1718-y
International development of four EORTC disease-specific quality of life questionnaires for patients with Hodgkin lymphoma, high- and low-grade non-Hodgkin lymphoma and chronic lymphocytic leukaemia
Abstract
Purpose: This paper describes the international, cross-cultural development of four disease-specific EORTC QoL questionnaires, to supplement the EORTC QLQ-C30, for patients with Hodgkin lymphoma (HL), high- or low-grade non-Hodgkin lymphoma (HG/LG-NHL), and CLL.
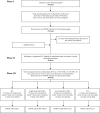
Methods: Questionnaire development was conducted according to guidelines from the EORTC Quality of Life Group. Phase I comprised generation of QoL issues relevant to patients. Phase II included operationalization and assessment of item relevance. In phase III, items were pretested in a cross-cultural sample.
Results: In Phase I, 75 issues were identified through focus groups and systematic literature searches. Interviews with 80 health-care professionals and 245 patients resulted in a provisional module of 38 items (phase II) representing items relevant for all or at least one of the four malignancies. In Phase III, this was tested in 337 patients from five European countries and resulted in a questionnaire with 27 items for HL (EORTC QLQ-HL27), 29 items for HG-NHL (EORTC QLQ-NHL-HG29), 20 items for LG-NHL (EORTC QLQ-NHL-LG20) and 17 items for CLL (EORTC QLQ-CLL17).
Conclusions: This study provides four new EORTC modules for use in clinical research and routine practice in conjunction with the EORTC QLQ-C30 for assessing QoL in patients with lymphoma and CLL.
Keywords: Chronic lymphocytic leukaemia; Hodgkin lymphoma; Non-Hodgkin lymphoma; Quality of life; Symptoms.
Conflict of interest statement
Conflict of interest
Fabio Efficace has received grants from Lundbeck, TEVA and Amgen, and has consultancy activities for Bristol-Myers Squibb, Seattle Genetics, TEVA and Incyte. The rest of the authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
-
- van de Schans SA, Issa DE, Visser O, Nooijen P, Huijgens PC, Karim-Kos HE, et al. Diverging trends in incidence and mortality, and improved survival of non-Hodgkin’s lymphoma, in the Netherlands, 1989–2007. Annals of Oncology: Official Journal of the European Society for Medical Oncology/ESMO. 2012;23(1):171–182. doi: 10.1093/annonc/mdr055. - DOI - PubMed
-
- Junlen HR, Peterson S, Kimby E, Lockmer S, Linden O, Nilsson-Ehle H, et al. Follicular lymphoma in Sweden: Nationwide improved survival in the rituximab era, particularly in elderly women: A Swedish Lymphoma Registry study. Leukemia: Official Journal of the Leukemia Society of America, Leukemia Research Fund, U. K. 2015;29(3):668–676. doi: 10.1038/leu.2014.251. - DOI - PubMed
-
- Mounier M, Bossard N, Remontet L, Belot A, Minicozzi P, De Angelis R, et al. Changes in dynamics of excess mortality rates and net survival after diagnosis of follicular lymphoma or diffuse large B-cell lymphoma: comparison between European population-based data (EUROCARE-5) Lancet Haematology. 2015;2(11):e481e491. doi: 10.1016/S2352-3026(15)00155-6. - DOI - PubMed
-
- Dutch Cancer Society . Cancer in the Netherlands until 2020, Trends and Prognoses [Kanker in Nederland tot 2020, Trends en prognoses] Amsterdam: KWF Kankerbestrijding; 2011.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical