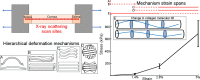
The hierarchical response of human corneal collagen to load
- PMID: 29128531
- PMCID: PMC5729024
- DOI: 10.1016/j.actbio.2017.11.015
The hierarchical response of human corneal collagen to load
Abstract
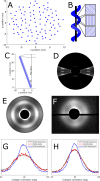
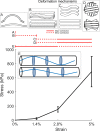
Fibrillar collagen in the human cornea is integral to its function as a transparent lens of precise curvature, and its arrangement is now well-characterised in the literature. While there has been considerable effort to incorporate fibrillar architecture into mechanical models of the cornea, the mechanical response of corneal collagen to small applied loads is not well understood. In this study the fibrillar and molecular response to tensile load was quantified using small and wide angle X-ray scattering (SAXS/WAXS), and digital image correlation (DIC) photography was used to calculate the local strain field that gave rise to the hierarchical changes. A molecular scattering model was used to calculate the tropocollagen tilt relative to the fibril axis and changes associated with applied strain. Changes were measured in the D-period, molecular tilt and the orientation and spacing of the fibrillar and molecular networks. These measurements were summarised into hierarchical deformation mechanisms, which were found to contribute at varying strains. The change in molecular tilt is indicative of a sub-fibrillar "spring-like" deformation mechanism, which was found to account for most of the applied strain under physiological and near-physiological loads. This deformation mechanism may play an important functional role in tissues rich in fibrils of high helical tilt, such as skin and cartilage.
Statement of significance: Collagen is the primary mediator of soft tissue biomechanics, and variations in its hierarchical structure convey the varying amounts of structural support necessary for organs to function normally. Here we have examined the structural response of corneal collagen to tensile load using X-rays to probe hierarchies ranging from molecular to fibrillar. We found a previously unreported deformation mechanism whereby molecules, which are helically arranged relative to the axis of their fibril, change in tilt akin to the manner in which a spring stretches. This "spring-like" mechanism accounts for a significant portion of the applied deformation at low strains (<3%). These findings will inform the future design of collagen-based artificial corneas being developed to address world-wide shortages of corneal donor tissue.
Keywords: Biomechanics; Collagen; Cornea; Microstructure; X-ray scattering.
Copyright © 2017 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures





References
-
- Sasaki N., Odajima S. Stress-strain curve and Young’s modulus of a collagen molecule as determined by the X-ray diffraction technique. J. Biomech. 1996;29:655–658. - PubMed
-
- Bruckner P. Suprastructures of extracellular matrices: paradigms of functions controlled by aggregates rather than molecules. Cell Tissue Res. 2010;339:7–18. - PubMed
-
- Kadler K.E., Baldock C., Bella J., Boot-Handford R.P. Collagens at a glance. J. Cell Sci. 2007;120:1955–1958. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

