Biallelic interferon regulatory factor 8 mutation: A complex immunodeficiency syndrome with dendritic cell deficiency, monocytopenia, and immune dysregulation
- PMID: 29128673
- PMCID: PMC5986711
- DOI: 10.1016/j.jaci.2017.08.044
Biallelic interferon regulatory factor 8 mutation: A complex immunodeficiency syndrome with dendritic cell deficiency, monocytopenia, and immune dysregulation
Abstract
Background: The homozygous K108E mutation of interferon regulatory factor 8 (IRF8) is reported to cause dendritic cell (DC) and monocyte deficiency. However, more widespread immune dysfunction is predicted from the multiple roles ascribed to IRF8 in immune cell development and function.
Objective: We sought to describe the effect on hematopoiesis and immunity of the compound heterozygous R83C/R291Q mutation of IRF8, which is present in a patient with recurrent viral infection, granuloproliferation, and intracerebral calcification.
Methods: Variant IRF8 alleles were identified by means of exome sequencing, and their function was tested by using reporter assays. The cellular phenotype was studied in detail by using flow cytometry, functional immunologic assay transcriptional profiling, and antigen receptor profiling.
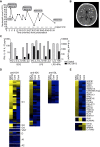
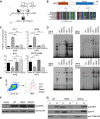
Results: Both mutations affected conserved residues, and R291Q is orthologous to R294, which is mutated in the BXH2 IRF8-deficient mouse. R83C showed reduced nuclear translocation, and neither mutant was able to regulate the Ets/IRF composite element or interferon-stimulated response element, whereas R291Q retained BATF/JUN interactions. DC deficiency and monocytopenia were observed in blood, dermis, and lung lavage fluid. Granulocytes were consistently increased, dysplastic, and hypofunctional. Natural killer cell development and maturation were arrested. TH1, TH17, and CD8+ memory T-cell differentiation was significantly reduced, and T cells did not express CXCR3. B-cell development was impaired, with fewer memory cells, reduced class-switching, and lower frequency and complexity of somatic hypermutation. Cell-specific gene expression was widely disturbed in interferon- and IRF8-regulated transcripts.
Conclusions: This analysis defines the clinical features of human biallelic IRF8 deficiency, revealing a complex immunodeficiency syndrome caused by DC and monocyte deficiency combined with widespread immune dysregulation.
Keywords: Interferon regulatory factor 8; dendritic cell; immunodeficiency; interferon; monocyte; myeloproliferation.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Tamura T., Yanai H., Savitsky D., Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol. 2008;26:535–584. - PubMed
-
- Kanno Y., Levi B.Z., Tamura T., Ozato K. Immune cell-specific amplification of interferon signaling by the IRF-4/8-PU.1 complex. J Interferon Cytokine Res. 2005;25:770–779. - PubMed
-
- Murphy T.L., Tussiwand R., Murphy K.M. Specificity through cooperation: BATF-IRF interactions control immune-regulatory networks. Nat Rev Immunol. 2013;13:499–509. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

