Raptor localization predicts prognosis and tamoxifen response in estrogen receptor-positive breast cancer
- PMID: 29128895
- PMCID: PMC5847064
- DOI: 10.1007/s10549-017-4508-x
Raptor localization predicts prognosis and tamoxifen response in estrogen receptor-positive breast cancer
Abstract
Purpose: Deregulated PI3K/mTOR signals can promote the growth of breast cancer and contribute to endocrine treatment resistance. This report aims to investigate raptor and its intracellular localization to further understand its role in ER-positive breast cancer.
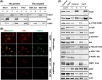
Methods: Raptor protein expression was evaluated by immunohistochemistry in 756 primary breast tumors from postmenopausal patients randomized to tamoxifen or no tamoxifen. In vitro, the MCF7 breast cancer cell line and tamoxifen-resistant MCF7 cells were studied to track the raptor signaling changes upon resistance, and raptor localization in ERα-positive cell lines was compared with that in ERα-negative cell lines.
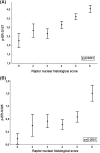
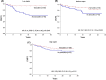
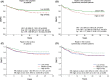
Results: Raptor protein expression in the nucleus was high in ER/PgR-positive and HER2-negative tumors with low grade, features associated with the luminal A subtype. Presence of raptor in the nucleus was connected with ERα signaling, here shown by a coupled increase of ERα phosphorylation at S167 and S305 with accumulation of nuclear raptor. In addition, the expression of ERα-activated gene products correlated with nuclear raptor. Similarly, in vitro we observed raptor in the nucleus of ERα-positive, but not of ER-negative cells. Interestingly, raptor localized to the nucleus could still be seen in tamoxifen-resistant MCF7 cells. The clinical benefit from tamoxifen was inversely associated with an increase of nuclear raptor. High cytoplasmic raptor expression indicated worse prognosis on long-term follow-up.
Conclusion: We present a connection between raptor localization to the nucleus and ERα-positive breast cancer, suggesting raptor as a player in stimulating the growth of the luminal A subtype and a possible target along with endocrine treatment.
Keywords: Endocrine resistance; Estrogen receptor (ER) α; Luminal A; Tamoxifen; mTOR.
Conflict of interest statement
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Ethical approval for the cohort was obtained from the Karolinska Institute Ethics Council, with an approved addition 02-01-2003. According to the approval, informed consent from the patients was not required.
Figures
References
-
- Miller TW, Hennessy BT, Gonzalez-Angulo AM, Fox EM, Mills GB, Chen H, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Investig. 2010;120(7):2406–2413. doi: 10.1172/JCI41680. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

