Anti-SEMA3A Antibody: A Novel Therapeutic Agent to Suppress Glioblastoma Tumor Growth
- PMID: 29129044
- PMCID: PMC6056981
- DOI: 10.4143/crt.2017.315
Anti-SEMA3A Antibody: A Novel Therapeutic Agent to Suppress Glioblastoma Tumor Growth
Abstract
Purpose: Glioblastoma (GBM) is classified as one of the most aggressive and lethal brain tumor. Great strides have been made in understanding the genomic and molecular underpinnings of GBM, which translated into development of new therapeutic approaches to combat such deadly disease. However, there are only few therapeutic agents that can effectively inhibit GBM invasion in a clinical framework. In an effort to address such challenges, we have generated anti-SEMA3A monoclonal antibody as a potential therapeutic antibody against GBM progression.
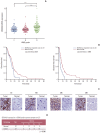
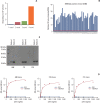
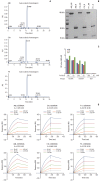
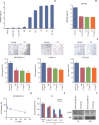
Materials and methods: We employed public glioma datasets, Repository of Molecular Brain Neoplasia Data and The Cancer Genome Atlas, to analyze SEMA3AmRNA expression in human GBM specimens. We also evaluated for protein expression level of SEMA3A via tissue microarray (TMA) analysis. Cell migration and proliferation kinetics were assessed in various GBM patient-derived cells (PDCs) and U87-MG cell-line for SEMA3A antibody efficacy. GBM patient-derived xenograft (PDX) models were generated to evaluate tumor inhibitory effect of anti-SEMA3A antibody in vivo.
Results: By combining bioinformatics and TMA analysis, we discovered that SEMA3A is highly expressed in human GBM specimens compared to non-neoplastic tissues. We developed three different anti-SEMA3A antibodies, in fully human IgG form, through screening phage-displayed synthetic antibody library using a classical panning method. Neutralization of SEMA3A significantly reduced migration and proliferation capabilities of PDCs and U87-MG cell line in vitro. In PDX models, treatment with anti-SEMA3A antibody exhibited notable tumor inhibitory effect through down-regulation of cellular proliferative kinetics and tumor-associated macrophages recruitment.
Conclusion: In present study, we demonstrated tumor inhibitory effect of SEMA3A antibody in GBM progression and present its potential relevance as a therapeutic agent in a clinical framework.
Keywords: Fully human antibody; Neutralization; Semaphorin-3A; Glioblastoma.
Conflict of interest statement
Conflict of interest relevant to this article was not reported.
Figures
Similar articles
-
Semaphorin 3A mediated brain tumor stem cell proliferation and invasion in EGFRviii mutant gliomas.BMC Cancer. 2020 Dec 10;20(1):1213. doi: 10.1186/s12885-020-07694-4. BMC Cancer. 2020. PMID: 33302912 Free PMC article.
-
Combined acetyl-11-keto-β-boswellic acid and radiation treatment inhibited glioblastoma tumor cells.PLoS One. 2018 Jul 3;13(7):e0198627. doi: 10.1371/journal.pone.0198627. eCollection 2018. PLoS One. 2018. PMID: 29969452 Free PMC article.
-
Resveratrol targeting of AKT and p53 in glioblastoma and glioblastoma stem-like cells to suppress growth and infiltration.J Neurosurg. 2017 May;126(5):1448-1460. doi: 10.3171/2016.1.JNS152077. Epub 2016 Jul 15. J Neurosurg. 2017. PMID: 27419830 Free PMC article.
-
Transcription factors in glioblastoma - Molecular pathogenesis and clinical implications.Biochim Biophys Acta Rev Cancer. 2022 Jan;1877(1):188667. doi: 10.1016/j.bbcan.2021.188667. Epub 2021 Dec 8. Biochim Biophys Acta Rev Cancer. 2022. PMID: 34894431 Review.
-
Ion channel expression and function in glioblastoma multiforme (GBM): pathophysiological mechanisms and therapeutic potential.Biochim Biophys Acta Mol Cell Res. 2025 Aug;1872(6):119982. doi: 10.1016/j.bbamcr.2025.119982. Epub 2025 May 5. Biochim Biophys Acta Mol Cell Res. 2025. PMID: 40328081 Review.
Cited by
-
Sphere-Forming Culture for Expanding Genetically Distinct Patient-Derived Glioma Stem Cells by Cellular Growth Rate Screening.Cancers (Basel). 2020 Feb 27;12(3):549. doi: 10.3390/cancers12030549. Cancers (Basel). 2020. PMID: 32120790 Free PMC article.
-
Development of an orthotopic medulloblastoma zebrafish model for rapid drug testing.Neuro Oncol. 2025 Mar 7;27(3):779-794. doi: 10.1093/neuonc/noae210. Neuro Oncol. 2025. PMID: 39383211 Free PMC article.
-
Semaphorin Regulation by the Chromatin Remodeler CHD7: An Emerging Genetic Interaction Shaping Neural Cells and Neural Crest in Development and Cancer.Front Cell Dev Biol. 2021 Apr 1;9:638674. doi: 10.3389/fcell.2021.638674. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33869187 Free PMC article. Review.
-
Neuropilin-1: a checkpoint target with unique implications for cancer immunology and immunotherapy.J Immunother Cancer. 2020 Jul;8(2):e000967. doi: 10.1136/jitc-2020-000967. J Immunother Cancer. 2020. PMID: 32675311 Free PMC article. Review.
-
Boric Acid Induces Oxidative Damage and Apoptosis Through SEMA3A/PLXNA1/NRP1 Signalling Pathway in U251 Glioblastoma Cell.J Cell Mol Med. 2025 May;29(9):e70578. doi: 10.1111/jcmm.70578. J Cell Mol Med. 2025. PMID: 40318008 Free PMC article.
References
-
- Adamson C, Kanu OO, Mehta AI, Di C, Lin N, Mattox AK, et al. Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs. 2009;18:1061–83. - PubMed
-
- Reardon DA, Wen PY. Therapeutic advances in the treatment of glioblastoma: rationale and potential role of targeted agents. Oncologist. 2006;11:152–64. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

