Lysophosphatidylcholine Regulates Sexual Stage Differentiation in the Human Malaria Parasite Plasmodium falciparum
- PMID: 29129376
- PMCID: PMC5733390
- DOI: 10.1016/j.cell.2017.10.020
Lysophosphatidylcholine Regulates Sexual Stage Differentiation in the Human Malaria Parasite Plasmodium falciparum
Abstract
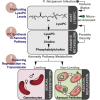
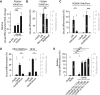
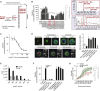
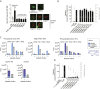
Transmission represents a population bottleneck in the Plasmodium life cycle and a key intervention target of ongoing efforts to eradicate malaria. Sexual differentiation is essential for this process, as only sexual parasites, called gametocytes, are infective to the mosquito vector. Gametocyte production rates vary depending on environmental conditions, but external stimuli remain obscure. Here, we show that the host-derived lipid lysophosphatidylcholine (LysoPC) controls P. falciparum cell fate by repressing parasite sexual differentiation. We demonstrate that exogenous LysoPC drives biosynthesis of the essential membrane component phosphatidylcholine. LysoPC restriction induces a compensatory response, linking parasite metabolism to the activation of sexual-stage-specific transcription and gametocyte formation. Our results reveal that malaria parasites can sense and process host-derived physiological signals to regulate differentiation. These data close a critical knowledge gap in parasite biology and introduce a major component of the sexual differentiation pathway in Plasmodium that may provide new approaches for blocking malaria transmission.
Keywords: Kennedy pathway; Plasmodium falciparum; environmental sensing; lysophosphatidylcholine; malaria; phospholipid metabolism; sexual differentiation; transmission.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures




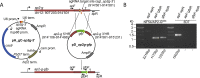
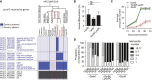
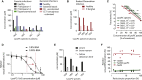


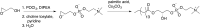
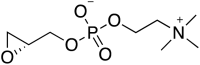
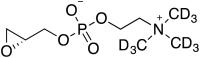
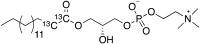

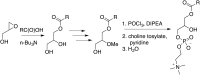
Comment in
-
Less Lipid, More Commitment.Cell. 2017 Dec 14;171(7):1474-1476. doi: 10.1016/j.cell.2017.11.044. Cell. 2017. PMID: 29245007 Free PMC article.
References
-
- Barnard G.A. Significance tests for 2 X 2 tables. Biometrika. 1947;34:123–138. - PubMed
-
- Billker O., Lindo V., Panico M., Etienne A.E., Paxton T., Dell A., Rogers M., Sinden R.E., Morris H.R. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature. 1998;392:289–292. - PubMed
-
- Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

