Adjuvant chemotherapy with or without bevacizumab in patients with resected non-small-cell lung cancer (E1505): an open-label, multicentre, randomised, phase 3 trial
- PMID: 29129443
- PMCID: PMC5789803
- DOI: 10.1016/S1470-2045(17)30691-5
Adjuvant chemotherapy with or without bevacizumab in patients with resected non-small-cell lung cancer (E1505): an open-label, multicentre, randomised, phase 3 trial
Abstract
Background: Adjuvant chemotherapy for resected early-stage non-small-cell lung cancer (NSCLC) provides a modest survival benefit. Bevacizumab, a monoclonal antibody directed against VEGF, improves outcomes when added to platinum-based chemotherapy in advanced-stage non-squamous NSCLC. We aimed to evaluate the addition of bevacizumab to adjuvant chemotherapy in early-stage resected NSCLC.
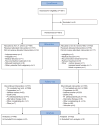
Methods: We did an open-label, randomised, phase 3 trial of adult patients (aged ≥18 years) with an Eastern Cooperative Oncology Group performance status of 0 or 1 and who had completely resected stage IB (≥4 cm) to IIIA (defined by the American Joint Committee on Cancer 6th edition) NSCLC. We enrolled patients from across the US National Clinical Trials Network, including patients from the Eastern Cooperative Oncology Group-American College of Radiology Imaging Network (ECOG-ACRIN) affiliates in Europe and from the Canadian Cancer Trials Group, within 6-12 weeks of surgery. The chemotherapy regimen for each patient was selected before randomisation and administered intravenously; it consisted of four 21-day cycles of cisplatin (75 mg/m2 on day 1 in all regimens) in combination with investigator's choice of vinorelbine (30 mg/m2 on days 1 and 8), docetaxel (75 mg/m2 on day 1), gemcitabine (1200 mg/m2 on days 1 and 8), or pemetrexed (500 mg/m2 on day 1). Patients in the bevacizumab group received bevacizumab 15 mg/kg intravenously every 21 days starting with cycle 1 of chemotherapy and continuing for 1 year. We randomly allocated patients (1:1) to group A (chemotherapy alone) or group B (chemotherapy plus bevacizumab), centrally, using permuted blocks sizes and stratified by chemotherapy regimen, stage of disease, histology, and sex. No one was masked to treatment assignment, except the Data Safety and Monitoring Committee. The primary endpoint was overall survival, analysed by intention to treat. This trial is registered with ClinicalTrials.gov, number NCT00324805.
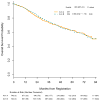
Findings: Between June 1, 2007, and Sept 20, 2013, 1501 patients were enrolled and randomly assigned to the two treatment groups: 749 to group A (chemotherapy alone) and 752 to group B (chemotherapy plus bevacizumab). 383 (26%) of 1458 patients (with complete staging information) had stage IB, 636 (44%) had stage II, and 439 (30%) had stage IIIA disease (stage of disease data were missing for 43 patients). Squamous cell histology was reported for 422 (28%) of 1501 patients. All four cisplatin-based chemotherapy regimens were used: 377 (25%) patients received vinorelbine, 343 (23%) received docetaxel, 283 (19%) received gemcitabine, and 497 (33%) received pemetrexed. At a median follow-up of 50·3 months (IQR 32·9-68·0), the estimated median overall survival in group A has not been reached, and in group B was 85·8 months (95% CI 74·9 to not reached); hazard ratio (group B vs group A) 0·99 (95% CI 0·82-1·19; p=0·90). Grade 3-5 toxicities of note (all attributions) that were reported more frequently in group B (the bevacizumab group) than in group A (chemotherapy alone) were overall worst grade (ie, all grade 3-5 toxicities; 496 [67%] of 738 in group A vs 610 [83%] of 735 in group B), hypertension (60 [8%] vs 219 [30%]), and neutropenia (241 [33%] vs 275 [37%]). The number of deaths on treatment did not differ between the groups (15 deaths in group A vs 19 in group B). Of these deaths, three in group A and ten in group B were considered at least possibly related to treatment.
Interpretation: Addition of bevacizumab to adjuvant chemotherapy did not improve overall survival for patients with surgically resected early-stage NSCLC. Bevacizumab does not have a role in this setting and should not be considered as an adjuvant therapy for patients with resected early-stage NSCLC.
Funding: National Cancer Institute of the National Institutes of Health.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
HW reports honoraria from Peregrine, honoraria from ACEA, grants and honoraria from Pfizer, honoraria from Helsinn, grants and honoraria from Genentech/Roche, grants from Clovis, grants from Exelixis, grants from AstraZeneca/Medimmune, grants from BMS, grants from Gilead, grants from Novartis, grants from Xcovery, grants from Celgene, grants from Pharmacyclics, grants from Lilly, outside the submitted work. SD reports research grants from NIH-NCTN to ECOG-ACRIN and grants from Genentech during the conduct of the study; personal fees from AstraZeneca, outside the submitted work. In addition, SD has a patent, Methods of Assessing Tumor Growth, for Dana-Farber Cancer Institute, Patent Application No. PCT/US2014/054821, pending. SK, following completion of the study and presentation at the World Lung Conference, left the clinical practice of Thoracic Surgery at the Montefiore Medical Center and joined Merck on November 28, 2016 as a Senior Principal Scientist in the Lung Oncology Group. SM reports personal fees from Clovis, grants and personal fees from Pfizer, grants from Amgen, personal fees and research funding from Bristol-Myers Squibb, research funding from Merck, research funding from Bayer, research funding from Janssen, grants from Celgene, outside the submitted work. RP received honoraria from Roche/Genentech for advisory boards and educational speaking engagements. TE reports personal frees from Genentech, outside the submitted work. LH reports personal fees from AbbVie advisory board, pro bono from Bristol Myers Squib advisory board, pro bono from Boehringer Ingelheim advisory board, personal fees from Lilly advisory board, personal fees from Genentech-Roche advisory board, payment to institution from Merck advisory board, pro bono from Xcovery consulting, pro bono from Bayer consulting, outside the submitted work. SR reports advisory board from Amgen, AstraZeneca, AbbVie, Boehringer Ingelheim, BMS, Lilly, Celgene, Genentech, and Novartis, outside the submitted work. JS reports research grants from National Cancer Institute during the conduct of the study; grants from Genentech, personal fees from Genentech, grants from Lilly, personal fees from Lilly, outside the submitted work. The other authors declared no conflicts of interest.
Figures





Comment in
-
Bevacizumab in adjuvant treatment of non-small-cell lung cancer.Lancet Oncol. 2017 Dec;18(12):1558-1560. doi: 10.1016/S1470-2045(17)30843-4. Epub 2017 Nov 9. Lancet Oncol. 2017. PMID: 29129445 No abstract available.
-
Adjuvant bevacizumab for resected non-small cell lung cancer: the end of an era?Transl Lung Cancer Res. 2018 Apr;7(Suppl 2):S179-S182. doi: 10.21037/tlcr.2018.03.24. Transl Lung Cancer Res. 2018. PMID: 29782560 Free PMC article. No abstract available.
References
-
- Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350(4):351–60. - PubMed
-
- Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352(25):2589–97. - PubMed
-
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7(9):719–27. - PubMed
-
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung Adjuvant Cisplatin Evaluation: A Pooled Analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UG1 CA233270/CA/NCI NIH HHS/United States
- UG1 CA189859/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- U10 CA180846/CA/NCI NIH HHS/United States
- UG1 CA189858/CA/NCI NIH HHS/United States
- U10 CA180847/CA/NCI NIH HHS/United States
- UG1 CA232760/CA/NCI NIH HHS/United States
- UG1 CA189830/CA/NCI NIH HHS/United States
- UG1 CA189863/CA/NCI NIH HHS/United States
- UG1 CA233340/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- U10 CA180863/CA/NCI NIH HHS/United States
- U10 CA180790/CA/NCI NIH HHS/United States
- U10 CA180799/CA/NCI NIH HHS/United States
- U10 CA180870/CA/NCI NIH HHS/United States
- U10 CA180816/CA/NCI NIH HHS/United States
- U10 CA180844/CA/NCI NIH HHS/United States
- U10 CA180864/CA/NCI NIH HHS/United States
- U10 CA180866/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

