CD38-NAD+Axis Regulates Immunotherapeutic Anti-Tumor T Cell Response
- PMID: 29129787
- PMCID: PMC5837048
- DOI: 10.1016/j.cmet.2017.10.006
CD38-NAD+Axis Regulates Immunotherapeutic Anti-Tumor T Cell Response
Abstract
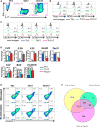
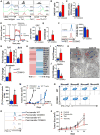
Heightened effector function and prolonged persistence, the key attributes of Th1 and Th17 cells, respectively, are key features of potent anti-tumor T cells. Here, we established ex vivo culture conditions to generate hybrid Th1/17 cells, which persisted long-term in vivo while maintaining their effector function. Using transcriptomics and metabolic profiling approaches, we showed that the enhanced anti-tumor property of Th1/17 cells was dependent on the increased NAD+-dependent activity of the histone deacetylase Sirt1. Pharmacological or genetic inhibition of Sirt1 activity impaired the anti-tumor potential of Th1/17 cells. Importantly, T cells with reduced surface expression of the NADase CD38 exhibited intrinsically higher NAD+, enhanced oxidative phosphorylation, higher glutaminolysis, and altered mitochondrial dynamics that vastly improved tumor control. Lastly, blocking CD38 expression improved tumor control even when using Th0 anti-tumor T cells. Thus, strategies targeting the CD38-NAD+ axis could increase the efficacy of anti-tumor adoptive T cell therapy.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures





Comment in
-
Metabolic Reprogramming via Targeting CD38 NADase Augments Adoptive T Cell Therapy.Cell Metab. 2018 Jan 9;27(1):3-5. doi: 10.1016/j.cmet.2017.12.014. Cell Metab. 2018. PMID: 29320709
References
-
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

