Psychological stress exposure to aged mice causes abnormal feeding patterns with changes in the bout number
- PMID: 29129830
- PMCID: PMC5723686
- DOI: 10.18632/aging.101320
Psychological stress exposure to aged mice causes abnormal feeding patterns with changes in the bout number
Abstract
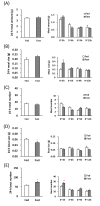
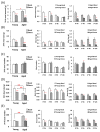
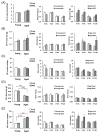
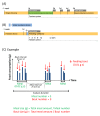
Stress responses are affected by aging. However, studies on stress-related changes in feeding patterns with aging subject are minimal. We investigated feeding patterns induced by two psychological stress models, revealing characteristics of stress-induced feeding patterns as "meal" and "bout" (defined as the minimum feeding behavior parameters) in aged mice. Feeding behaviors of C57BL/6J mice were monitored for 24 h by an automatic monitoring device. Novelty stress reduced the meal amount over the 24 h in both young and aged mice, but as a result of a time course study it was persistent in aged mice. In addition, the decreased bout number was more pronounced in aged mice than in young mice. The 24-h meal and bout parameters did not change in either the young or aged mice following water avoidance stress (WAS). However, the meal amount and bout number increased in aged mice for 0-6 h after WAS exposure but remained unchanged in young mice. Our findings suggest that changes in bout number may lead to abnormal stress-related feeding patterns and may be one tool for evaluating eating abnormality in aged mice.
Keywords: aged mice; bout; meal microstructure; meal pattern; stress.
Conflict of interest statement
The all authors are employees of Tsumura & Co. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
Figures

Similar articles
-
Peptide YY induces characteristic meal patterns of aged mice.Horm Behav. 2017 Nov;96:62-68. doi: 10.1016/j.yhbeh.2017.09.003. Horm Behav. 2017. PMID: 28916138
-
Meal pattern alterations associated with intermittent fasting for weight loss are normalized after high-fat diet re-feeding.Physiol Behav. 2017 May 15;174:49-56. doi: 10.1016/j.physbeh.2017.02.046. Epub 2017 Mar 3. Physiol Behav. 2017. PMID: 28263771
-
Influence of Aging and Gender Differences on Feeding Behavior and Ghrelin-Related Factors during Social Isolation in Mice.PLoS One. 2015 Oct 8;10(10):e0140094. doi: 10.1371/journal.pone.0140094. eCollection 2015. PLoS One. 2015. PMID: 26448274 Free PMC article.
-
A Framework for Developing Translationally Relevant Animal Models of Stress-Induced Changes in Eating Behavior.Biol Psychiatry. 2022 May 15;91(10):888-897. doi: 10.1016/j.biopsych.2021.06.020. Epub 2021 Jul 3. Biol Psychiatry. 2022. PMID: 34433512 Free PMC article. Review.
-
The influence of feeding and fasting on plasma metabolites in the dogfish shark (Squalus acanthias).Comp Biochem Physiol A Mol Integr Physiol. 2010 Apr;155(4):435-44. doi: 10.1016/j.cbpa.2009.09.006. Epub 2009 Sep 24. Comp Biochem Physiol A Mol Integr Physiol. 2010. PMID: 19782147 Review.
Cited by
-
Systematic analysis of differential rhythmic liver gene expression mediated by the circadian clock and feeding rhythms.Proc Natl Acad Sci U S A. 2021 Jan 19;118(3):e2015803118. doi: 10.1073/pnas.2015803118. Proc Natl Acad Sci U S A. 2021. PMID: 33452134 Free PMC article.
-
The feeding microstructure of male and female mice.PLoS One. 2021 Feb 4;16(2):e0246569. doi: 10.1371/journal.pone.0246569. eCollection 2021. PLoS One. 2021. PMID: 33539467 Free PMC article.
-
Ghrelin Enhancer, the Latest Evidence of Rikkunshito.Front Nutr. 2021 Dec 9;8:761631. doi: 10.3389/fnut.2021.761631. eCollection 2021. Front Nutr. 2021. PMID: 34957179 Free PMC article. Review.
-
Involvement of Ghrelin Dynamics in Stress-Induced Eating Disorder: Effects of Sex and Aging.Int J Mol Sci. 2021 Oct 28;22(21):11695. doi: 10.3390/ijms222111695. Int J Mol Sci. 2021. PMID: 34769125 Free PMC article. Review.
References
-
- Casper RC. Depression and eating disorders. Depress Anxiety. 1998;8(Suppl 1):96–104. https://doi.org/10.1002/(SICI)1520-6394(1998)8:1+<96::AID-DA15>3.0.CO;2-4 - DOI - PubMed
-
- Kass AE, Kolko RP, Wilfley DE. Psychological treatments for eating disorders. Curr Opin Psychiatry. 2013;26:549–55. https://doi.org/10.1097/YCO.0b013e328365a30e - DOI - PMC - PubMed
-
- Jiang SZ, Eiden LE. Activation of the HPA axis and depression of feeding behavior induced by restraint stress are separately regulated by PACAPergic neurotransmission in the mouse. Stress. 2016;19:374–82. https://doi.org/10.1080/10253890.2016.1174851 - DOI - PMC - PubMed
-
- Harris RB. Chronic and acute effects of stress on energy balance: are there appropriate animal models? Am J Physiol Regul Integr Comp Physiol. 2015;308:R250–65. https://doi.org/10.1152/ajpregu.00361.2014 - DOI - PMC - PubMed
-
- Saegusa Y, Takeda H, Muto S, Nakagawa K, Ohnishi S, Sadakane C, Nahata M, Hattori T, Asaka M. Decreased plasma ghrelin contributes to anorexia following novelty stress. Am J Physiol Endocrinol Metab. 2011;301:E685–96. https://doi.org/10.1152/ajpendo.00121.2011 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

