Prostaglandin terminal synthases as novel therapeutic targets
- PMID: 29129850
- PMCID: PMC5743848
- DOI: 10.2183/pjab.93.044
Prostaglandin terminal synthases as novel therapeutic targets
Abstract
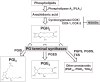
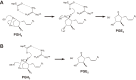
Non-steroidal anti-inflammatory drugs (NSAIDs) exert their anti-inflammatory and anti-tumor effects by reducing prostaglandin (PG) production via the inhibition of cyclooxygenase (COX). However, the gastrointestinal, renal and cardiovascular side effects associated with the pharmacological inhibition of the COX enzymes have focused renewed attention onto other potential targets for NSAIDs. PGH2, a COX metabolite, is converted to each PG species by species-specific PG terminal synthases. Because of their potential for more selective modulation of PG production, PG terminal synthases are now being investigated as a novel target for NSAIDs. In this review, I summarize the current understanding of PG terminal synthases, with a focus on microsomal PGE synthase-1 (mPGES-1) and PGI synthase (PGIS). mPGES-1 and PGIS cooperatively exacerbate inflammatory reactions but have opposing effects on carcinogenesis. mPGES-1 and PGIS are expected to be attractive alternatives to COX as therapeutic targets for several diseases, including inflammatory diseases and cancer.
Keywords: NSAIDs; carcinogenesis; inflammatory reaction; prostacyclin; prostaglandin.
Figures
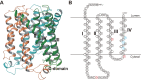

Similar articles
-
Genetic-deletion of Cyclooxygenase-2 Downstream Prostacyclin Synthase Suppresses Inflammatory Reactions but Facilitates Carcinogenesis, unlike Deletion of Microsomal Prostaglandin E Synthase-1.Sci Rep. 2015 Nov 27;5:17376. doi: 10.1038/srep17376. Sci Rep. 2015. PMID: 26611322 Free PMC article.
-
Dual acting anti-inflammatory drugs: a reappraisal.Pharmacol Res. 2001 Dec;44(6):437-50. doi: 10.1006/phrs.2001.0872. Pharmacol Res. 2001. PMID: 11735348 Review.
-
Perspective of microsomal prostaglandin E2 synthase-1 as drug target in inflammation-related disorders.Biochem Pharmacol. 2015 Nov 1;98(1):1-15. doi: 10.1016/j.bcp.2015.06.022. Epub 2015 Jun 27. Biochem Pharmacol. 2015. PMID: 26123522 Review.
-
Prostacyclin Synthase as an Ambivalent Regulator of Inflammatory Reactions.Biol Pharm Bull. 2022;45(8):979-984. doi: 10.1248/bpb.b22-00370. Biol Pharm Bull. 2022. PMID: 35908907 Review.
-
Relationship of the Topological Distances and Activities between mPGES-1 and COX-2 versus COX-1: Implications of the Different Post-Translational Endoplasmic Reticulum Organizations of COX-1 and COX-2.Biochemistry. 2015 Jun 16;54(23):3707-15. doi: 10.1021/acs.biochem.5b00339. Epub 2015 Jun 3. Biochemistry. 2015. PMID: 25988363
Cited by
-
Phagocytes produce prostaglandin E2 in response to cytosolic Listeria monocytogenes.PLoS Pathog. 2021 Sep 23;17(9):e1009493. doi: 10.1371/journal.ppat.1009493. eCollection 2021 Sep. PLoS Pathog. 2021. PMID: 34555127 Free PMC article.
-
Prostanoids and Resolution of Inflammation - Beyond the Lipid-Mediator Class Switch.Front Immunol. 2021 Jul 12;12:714042. doi: 10.3389/fimmu.2021.714042. eCollection 2021. Front Immunol. 2021. PMID: 34322137 Free PMC article. Review.
-
Prostacyclin synthase deficiency exacerbates systemic inflammatory responses in lipopolysaccharide-induced septic shock in mice.Inflamm Res. 2024 Aug;73(8):1349-1358. doi: 10.1007/s00011-024-01902-8. Epub 2024 Jun 4. Inflamm Res. 2024. PMID: 38832966
-
Expression of Enzymes Associated with Prostaglandin Synthesis in Equine Conceptuses.Animals (Basel). 2021 Apr 20;11(4):1180. doi: 10.3390/ani11041180. Animals (Basel). 2021. PMID: 33924239 Free PMC article.
-
Spatiotemporally distinct roles of cyclooxygenase-1 and cyclooxygenase-2 at fetomaternal interface in mice.JCI Insight. 2024 Aug 27;9(19):e181865. doi: 10.1172/jci.insight.181865. JCI Insight. 2024. PMID: 39377223 Free PMC article.
References
-
- Narumiya S. (1994) Prostanoid receptors: structure, function, and distribution. Ann. N. Y. Acad. Sci. 744, 126–138. - PubMed
-
- Murakami M., Taketomi Y., Miki Y., Sato H., Hirabayashi T., Yamamoto K. (2011) Recent progress in phospholipase A2 research: from cells to animals to humans. Prog. Lipid Res. 50, 152–192. - PubMed
-
- Smith E.L., DeWitt D.L., Garavito R.M. (2000) Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 69, 145–182. - PubMed
-
- Hara, S. (2015) Prostaglandin terminal synthases as novel drug targets. In Bioactive Lipid Mediators (eds. Yokomizo, T. and Murakami, M.). Springer Japan, Tokyo, pp. 43–57.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

