Dengue virus-reactive CD8+ T cells mediate cross-protection against subsequent Zika virus challenge
- PMID: 29129917
- PMCID: PMC5682281
- DOI: 10.1038/s41467-017-01669-z
Dengue virus-reactive CD8+ T cells mediate cross-protection against subsequent Zika virus challenge
Abstract
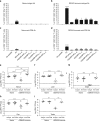
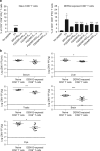
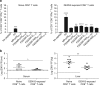
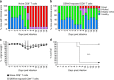
Zika virus (ZIKV) and dengue virus (DENV) are antigenically related flaviviruses that share cross-reactivity in antibody and T cell responses, and co-circulate in increasing numbers of countries. Whether pre-existing DENV immunity can cross-protect or enhance ZIKV infection during sequential infection of the same host is unknown. Here, we show that DENV-immune Ifnar1 -/- or wild-type C57BL/6 mice infected with ZIKV have cross-reactive immunity to subsequent ZIKV infection and pathogenesis. Adoptive transfer and cell depletion studies demonstrate that DENV-immune CD8+ T cells predominantly mediate cross-protective responses to ZIKV. In contrast, passive transfer studies suggest that DENV-immune serum does not protect against ZIKV infection. Thus, CD8+ T cell immunity generated during primary DENV infection can confer protection against secondary ZIKV infection in mice. Further optimization of current DENV vaccines for T cell responses might confer cross-protection and prevent antibody-mediated enhancement of ZIKV infection.
Conflict of interest statement
The authors declare that M.S.D. is a consultant for Inbios, Visterra, Aviana, and Takeda Pharmaceuticals and on the Scientific Advisory Boards of Moderna and OvaGene. The remaining authors declare no competing financial interests.
Figures

Similar articles
-
Cross-reactive Dengue virus-specific CD8+ T cells protect against Zika virus during pregnancy.Nat Commun. 2018 Aug 2;9(1):3042. doi: 10.1038/s41467-018-05458-0. Nat Commun. 2018. PMID: 30072692 Free PMC article.
-
Cross-Reactivity and Anti-viral Function of Dengue Capsid and NS3-Specific Memory T Cells Toward Zika Virus.Front Immunol. 2018 Oct 1;9:2225. doi: 10.3389/fimmu.2018.02225. eCollection 2018. Front Immunol. 2018. PMID: 30327651 Free PMC article.
-
Identification of Zika virus epitopes reveals immunodominant and protective roles for dengue virus cross-reactive CD8+ T cells.Nat Microbiol. 2017 Mar 13;2:17036. doi: 10.1038/nmicrobiol.2017.36. Nat Microbiol. 2017. PMID: 28288094 Free PMC article.
-
Cross-Reactive T Cell Immunity to Dengue and Zika Viruses: New Insights Into Vaccine Development.Front Immunol. 2019 Jun 11;10:1316. doi: 10.3389/fimmu.2019.01316. eCollection 2019. Front Immunol. 2019. PMID: 31244855 Free PMC article. Review.
-
Modulation of Dengue/Zika Virus Pathogenicity by Antibody-Dependent Enhancement and Strategies to Protect Against Enhancement in Zika Virus Infection.Front Immunol. 2018 Apr 23;9:597. doi: 10.3389/fimmu.2018.00597. eCollection 2018. Front Immunol. 2018. PMID: 29740424 Free PMC article. Review.
Cited by
-
Protective and enhancing interactions among dengue viruses 1-4 and Zika virus.Curr Opin Virol. 2020 Aug;43:59-70. doi: 10.1016/j.coviro.2020.08.006. Epub 2020 Sep 24. Curr Opin Virol. 2020. PMID: 32979816 Free PMC article. Review.
-
Repeated exposure to dengue virus elicits robust cross neutralizing antibodies against Zika virus in residents of Northeastern Thailand.Sci Rep. 2021 May 5;11(1):9634. doi: 10.1038/s41598-021-88933-x. Sci Rep. 2021. PMID: 33953258 Free PMC article.
-
Concomitant Transmission of Dengue, Chikungunya, and Zika Viruses in Brazil: Clinical and Epidemiological Findings From Surveillance for Acute Febrile Illness.Clin Infect Dis. 2019 Sep 27;69(8):1353-1359. doi: 10.1093/cid/ciy1083. Clin Infect Dis. 2019. PMID: 30561554 Free PMC article.
-
Cross-Reactive Immunity among Five Medically Important Mosquito-Borne Flaviviruses Related to Human Diseases.Viruses. 2022 Jun 2;14(6):1213. doi: 10.3390/v14061213. Viruses. 2022. PMID: 35746683 Free PMC article. Review.
-
In-depth characterization of congenital Zika syndrome in immunocompetent mice: Antibody-dependent enhancement and an antiviral peptide therapy.EBioMedicine. 2019 Jun;44:516-529. doi: 10.1016/j.ebiom.2019.05.014. Epub 2019 May 23. EBioMedicine. 2019. PMID: 31130472 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

