Intratumoral CD40 activation and checkpoint blockade induces T cell-mediated eradication of melanoma in the brain
- PMID: 29129918
- PMCID: PMC5682289
- DOI: 10.1038/s41467-017-01572-7
Intratumoral CD40 activation and checkpoint blockade induces T cell-mediated eradication of melanoma in the brain
Abstract
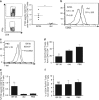
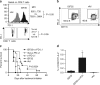
CD40 agonists bind the CD40 molecule on antigen-presenting cells and activate them to prime tumor-specific CD8+ T cell responses. Here, we study the antitumor activity and mechanism of action of a nonreplicating adenovirus encoding a chimeric, membrane-bound CD40 ligand (ISF35). Intratumoral administration of ISF35 in subcutaneous B16 melanomas generates tumor-specific, CD8+ T cells that express PD-1 and suppress tumor growth. Combination therapy of ISF35 with systemic anti-PD-1 generates greater antitumor activity than each respective monotherapy. Triple combination of ISF35, anti-PD-1, and anti-CTLA-4 results in complete eradication of injected and noninjected subcutaneous tumors, as well as melanoma tumors in the brain. Therapeutic efficacy is associated with increases in the systemic level of tumor-specific CD8+ T cells, and an increased ratio of intratumoral CD8+ T cells to CD4+ Tregs. These results provide a proof of concept of systemic antitumor activity after intratumoral CD40 triggering with ISF35 in combination with checkpoint blockade for multifocal cancer, including the brain.
Conflict of interest statement
M.J.C. is an employee of Memgen and inventor on patents and patent applications concerning the composition of matter and use of ISF35 for cancer therapy. W.W.O., M.Si., P.H. and M.J.C. are the authors and inventors on U.S. patent application “Methods and therapeutic combinations for treating tumors” No. 15/500,618 filed on 31 July 2015, concerning the use of ISF35 for cancer therapy. The remaining authors declare no competing financial interests.
Figures





Similar articles
-
Anti-PD-1/anti-CTLA-4 efficacy in melanoma brain metastases depends on extracranial disease and augmentation of CD8+ T cell trafficking.Proc Natl Acad Sci U S A. 2018 Feb 13;115(7):E1540-E1549. doi: 10.1073/pnas.1714089115. Epub 2018 Jan 31. Proc Natl Acad Sci U S A. 2018. PMID: 29386395 Free PMC article.
-
CD40 cross-linking bypasses the absolute requirement for CD4 T cells during immunization with melanoma antigen gene-modified dendritic cells.Cancer Res. 2001 Dec 15;61(24):8787-93. Cancer Res. 2001. PMID: 11751400
-
Interference of CD40L-mediated tumor immunotherapy by oncolytic vesicular stomatitis virus.Hum Gene Ther. 2010 Apr;21(4):439-50. doi: 10.1089/hum.2009.143. Hum Gene Ther. 2010. PMID: 19922169 Free PMC article.
-
Tumor regression induced by intratumor administration of adenovirus vector expressing CD40 ligand and naive dendritic cells.Cancer Res. 2000 Nov 15;60(22):6391-5. Cancer Res. 2000. PMID: 11103803
-
Facts and Hopes of CD40 Agonists in Cancer Immunotherapy.Clin Cancer Res. 2025 Jun 3;31(11):2079-2087. doi: 10.1158/1078-0432.CCR-24-1660. Clin Cancer Res. 2025. PMID: 40117130 Review.
Cited by
-
Adrenergic receptor signaling regulates the CD40-receptor mediated anti-tumor immunity.Front Immunol. 2023 Mar 15;14:1141712. doi: 10.3389/fimmu.2023.1141712. eCollection 2023. Front Immunol. 2023. PMID: 37006295 Free PMC article.
-
Intratumoral expression of IL-12 and CD40 ligand (CD154) from plasmids generates antitumor responses that eliminate tumoral T regs.Sci Rep. 2025 Jul 8;15(1):24439. doi: 10.1038/s41598-025-09535-5. Sci Rep. 2025. PMID: 40628928 Free PMC article.
-
The Role of CD4 T Cell Help in CD8 T Cell Differentiation and Function During Chronic Infection and Cancer.Immune Netw. 2023 Oct 23;23(5):e41. doi: 10.4110/in.2023.23.e41. eCollection 2023 Oct. Immune Netw. 2023. PMID: 37970230 Free PMC article. Review.
-
Nanomaterials-Mediated Co-Stimulation of Toll-Like Receptors and CD40 for Antitumor Immunity.Adv Mater. 2022 Nov;34(47):e2207486. doi: 10.1002/adma.202207486. Epub 2022 Oct 17. Adv Mater. 2022. PMID: 36121735 Free PMC article.
-
Concepts for agonistic targeting of CD40 in immuno-oncology.Hum Vaccin Immunother. 2020;16(2):377-387. doi: 10.1080/21645515.2019.1653744. Epub 2019 Sep 5. Hum Vaccin Immunother. 2020. PMID: 31403344 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

