Increasing the Fungicidal Action of Amphotericin B by Inhibiting the Nitric Oxide-Dependent Tolerance Pathway
- PMID: 29129987
- PMCID: PMC5654257
- DOI: 10.1155/2017/4064628
Increasing the Fungicidal Action of Amphotericin B by Inhibiting the Nitric Oxide-Dependent Tolerance Pathway
Abstract
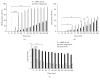
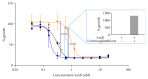
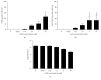
Amphotericin B (AmB) induces oxidative and nitrosative stresses, characterized by production of reactive oxygen and nitrogen species, in fungi. Yet, how these toxic species contribute to AmB-induced fungal cell death is unclear. We investigated the role of superoxide and nitric oxide radicals in AmB's fungicidal activity in Saccharomyces cerevisiae, using a digital microfluidic platform, which enabled monitoring individual cells at a spatiotemporal resolution, and plating assays. The nitric oxide synthase inhibitor L-NAME was used to interfere with nitric oxide radical production. L-NAME increased and accelerated AmB-induced accumulation of superoxide radicals, membrane permeabilization, and loss of proliferative capacity in S. cerevisiae. In contrast, the nitric oxide donor S-nitrosoglutathione inhibited AmB's action. Hence, superoxide radicals were important for AmB's fungicidal action, whereas nitric oxide radicals mediated tolerance towards AmB. Finally, also the human pathogens Candida albicans and Candida glabrata were more susceptible to AmB in the presence of L-NAME, pointing to the potential of AmB-L-NAME combination therapy to treat fungal infections.
Figures





Similar articles
-
Fluorescence studies on the molecular action of amphotericin B on susceptible and resistant fungal cells.Biochemistry. 1996 Jun 18;35(24):7983-92. doi: 10.1021/bi952910c. Biochemistry. 1996. PMID: 8672502
-
ROS formation is a differential contributory factor to the fungicidal action of Amphotericin B and Micafungin in Candida albicans.Int J Med Microbiol. 2017 Jun;307(4-5):241-248. doi: 10.1016/j.ijmm.2017.03.005. Epub 2017 Apr 4. Int J Med Microbiol. 2017. PMID: 28412040
-
Enhancement effect of N-methyl-N″-dodecylguanidine on the vacuole-targeting fungicidal activity of amphotericin B against the pathogenic fungus Candida albicans.J Antibiot (Tokyo). 2011 Jul;64(7):469-74. doi: 10.1038/ja.2011.31. Epub 2011 Apr 27. J Antibiot (Tokyo). 2011. PMID: 21522157
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
-
Antifungal drug resistance in pathogenic fungi.Med Mycol. 1998;36 Suppl 1:119-28. Med Mycol. 1998. PMID: 9988500 Review.
Cited by
-
Multiplex Analysis to Unravel the Mode of Antifungal Activity of the Plant Defensin HsAFP1 in Single Yeast Cells.Int J Mol Sci. 2022 Jan 28;23(3):1515. doi: 10.3390/ijms23031515. Int J Mol Sci. 2022. PMID: 35163438 Free PMC article.
-
Functional Analyses of the Bacillus velezensis HMB26553 Genome Provide Evidence That Its Genes Are Potentially Related to the Promotion of Plant Growth and Prevention of Cotton Rhizoctonia Damping-Off.Cells. 2023 May 2;12(9):1301. doi: 10.3390/cells12091301. Cells. 2023. PMID: 37174701 Free PMC article.
-
A Review: Antimicrobial Therapy for Human Pythiosis.Antibiotics (Basel). 2022 Mar 26;11(4):450. doi: 10.3390/antibiotics11040450. Antibiotics (Basel). 2022. PMID: 35453202 Free PMC article. Review.
-
Dielectrophoresis-Based Selective Droplet Extraction Microfluidic Device for Single-Cell Analysis.Micromachines (Basel). 2023 Mar 22;14(3):706. doi: 10.3390/mi14030706. Micromachines (Basel). 2023. PMID: 36985113 Free PMC article.
-
Antifungals: From Pharmacokinetics to Clinical Practice.Antibiotics (Basel). 2023 May 9;12(5):884. doi: 10.3390/antibiotics12050884. Antibiotics (Basel). 2023. PMID: 37237787 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

