Protein S-Nitrosylation: Determinants of Specificity and Enzymatic Regulation of S-Nitrosothiol-Based Signaling
- PMID: 29130312
- PMCID: PMC6391618
- DOI: 10.1089/ars.2017.7403
Protein S-Nitrosylation: Determinants of Specificity and Enzymatic Regulation of S-Nitrosothiol-Based Signaling
Abstract
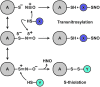
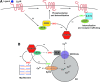
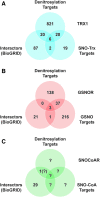
Significance: Protein S-nitrosylation, the oxidative modification of cysteine by nitric oxide (NO) to form protein S-nitrosothiols (SNOs), mediates redox-based signaling that conveys, in large part, the ubiquitous influence of NO on cellular function. S-nitrosylation regulates protein activity, stability, localization, and protein-protein interactions across myriad physiological processes, and aberrant S-nitrosylation is associated with diverse pathophysiologies. Recent Advances: It is recently recognized that S-nitrosylation endows S-nitroso-protein (SNO-proteins) with S-nitrosylase activity, that is, the potential to trans-S-nitrosylate additional proteins, thereby propagating SNO-based signals, analogous to kinase-mediated signaling cascades. In addition, it is increasingly appreciated that cellular S-nitrosylation is governed by dynamically coupled equilibria between SNO-proteins and low-molecular-weight SNOs, which are controlled by a growing set of enzymatic denitrosylases comprising two main classes (high and low molecular weight). S-nitrosylases and denitrosylases, which together control steady-state SNO levels, may be identified with distinct physiology and pathophysiology ranging from cardiovascular and respiratory disorders to neurodegeneration and cancer.
Critical issues: The target specificity of protein S-nitrosylation and the stability and reactivity of protein SNOs are determined substantially by enzymatic machinery comprising highly conserved transnitrosylases and denitrosylases. Understanding the differential functionality of SNO-regulatory enzymes is essential, and is amenable to genetic and pharmacological analyses, read out as perturbation of specific equilibria within the SNO circuitry.
Future directions: The emerging picture of NO biology entails equilibria among potentially thousands of different SNOs, governed by denitrosylases and nitrosylases. Thus, to elucidate the operation and consequences of S-nitrosylation in cellular contexts, studies should consider the roles of SNO-proteins as both targets and transducers of S-nitrosylation, functioning according to enzymatically governed equilibria.
Keywords: S-nitrosylase; S-nitrosylation; denitrosylation; nitric oxide; redox signaling.
Figures



References
-
- Andre FR, dos Santos PF, and Rando DG. Theoretical studies of the role of C-terminal cysteines in the process of S-nitrosylation of human Src kinases. J Mol Model 22: 23, 2016 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

