UBC-Nepal expedition: acclimatization to high-altitude increases spinal motoneurone excitability during fatigue in humans
- PMID: 29130497
- PMCID: PMC6068218
- DOI: 10.1113/JP274872
UBC-Nepal expedition: acclimatization to high-altitude increases spinal motoneurone excitability during fatigue in humans
Abstract
Key points: Acute exposure and acclimatization to hypoxia are associated with an impairment and partial recovery, respectively, of the capability of the central nervous system to drive muscles during prolonged efforts. Motoneurones play a vital role in muscle contraction and in fatigue, although the effect of hypoxia on motoneurone excitability during exercise has not been assessed in humans. We studied the impact of fatigue on motoneurone excitability in normoxia, acute and chronic exposure (5050 m) to hypoxia. Performance was worse in acute hypoxia but recovered to the normoxic standard in chronic hypoxia, in parallel with an increased excitability of the motoneurones compared to acute exposure to hypoxia. These findings reveal that prolonged hypoxia causes a heightened motoneurone responsiveness during fatiguing exercise; such an adaptation might favour the restoration of performance where low pressures of oxygen are chronically present.
Abstract: The fatigue-induced failure of the motor cortex to drive muscles maximally increases in acute hypoxia (AH) compared to normoxia (N) but improves with acclimatization (chronic hypoxia; CH). Despite their importance to muscle output, it is unknown how locomotor motoneurones in humans are affected by hypoxia and acclimatization. Eleven participants performed 16 min of submaximal [25% maximal torque (maximal voluntary contraction, MVC)] intermittent isometric elbow flexions in N, AH (environmental chamber) and CH (7-14 days at 5050 m) (PI O2 = 140, 74 and 76 mmHg, respectively). For each minute of the fatigue protocol, motoneurone responsiveness was measured with cervicomedullary stimulation delivered 100 ms after transcranial magnetic stimulation (TMS) used to transiently silence voluntary drive. Every 2 min, cortical voluntary activation (cVA) was measured with TMS. After the task, MVC torque declined more in AH (∼20%) than N and CH (∼11% and 14%, respectively, P < 0.05), with no differences between N and CH. cVA was lower in AH than N and CH at baseline (∼92%, 95% and 95%, respectively) and at the end of the protocol (∼82%, 90% and 90%, P < 0.05). During the fatiguing task, motoneurone excitability in N and AH declined to ∼65% and 40% of the baseline value (P < 0.05). In CH, motoneurone excitability did not decline and, late in the protocol, was ∼40% higher compared to AH (P < 0.05). These novel data reveal that acclimatization to hypoxia leads to a heightened motoneurone responsiveness during fatiguing exercise. Positive spinal and supraspinal adaptations during extended periods at altitude might therefore play a vital role for the restoration of performance after acclimatization to hypoxia.
Keywords: central fatigue; cervicomedullary motor evoked potential; hypoxia.
© 2017 The Authors. The Journal of Physiology © 2017 The Physiological Society.
Figures


 ), acute hypoxia (AH, ■) and chronic hypoxia (CH,▼). A, elbow flexor torque obtained from the last 25% MVC contraction of each minute. B, integrated EMG from the last contraction of each minute expressed as a percentage of the value obtained during baseline contractions at 25% MVC. Torque and iEMG were targeted accurately throughout the protocol and did not differ across sessions (P = 0.60 and P = 0.84 for torque and iEMG, respectively).
), acute hypoxia (AH, ■) and chronic hypoxia (CH,▼). A, elbow flexor torque obtained from the last 25% MVC contraction of each minute. B, integrated EMG from the last contraction of each minute expressed as a percentage of the value obtained during baseline contractions at 25% MVC. Torque and iEMG were targeted accurately throughout the protocol and did not differ across sessions (P = 0.60 and P = 0.84 for torque and iEMG, respectively).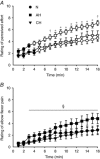
 ), acute hypoxia (AH, ■) and chronic hypoxia (CH,▼). Open symbols represent data points significantly higher than baseline values. Rating of perceived effort (A) and perceived pain in the elbow flexors (B) were collected during the last contraction of each minute at 25% iEMG. During the second half of the protocol, the rating of perceived effort was higher in AH compared to N (*
P < 0.05) or both N and CH (†
P < 0.05). For rating of elbow flexor pain, data were pooled across sessions (no main effect of session, nor a session × time interaction) and post hoc testing on the main effect of time revealed an increase from the 3rd min onward (§
P < 0.05).
), acute hypoxia (AH, ■) and chronic hypoxia (CH,▼). Open symbols represent data points significantly higher than baseline values. Rating of perceived effort (A) and perceived pain in the elbow flexors (B) were collected during the last contraction of each minute at 25% iEMG. During the second half of the protocol, the rating of perceived effort was higher in AH compared to N (*
P < 0.05) or both N and CH (†
P < 0.05). For rating of elbow flexor pain, data were pooled across sessions (no main effect of session, nor a session × time interaction) and post hoc testing on the main effect of time revealed an increase from the 3rd min onward (§
P < 0.05).
 ), acute hypoxia (AH, ■) and chronic hypoxia (CH,▼). Open symbols represent data points significantly lower than baseline values. Area of M
max (A) and area of CMEP (B) normalized to the area of the M
max evoked during the same contraction each minute, expressed as a percentage of the baseline value. C, absolute values of cortical voluntary activation measured at baseline (BL) and every 2 min during the fatiguing protocol. At the 10th, 11th, 13th and 15th min, CMEP area was lower in AH compared to CH (#
P < 0.05). Cortical voluntary activation was lower at BL and the 8th and 16th min in AH compared to both N and CH (†
P < 0.05), and at the 14th min in AH relative to CH (#
P < 0.05).
), acute hypoxia (AH, ■) and chronic hypoxia (CH,▼). Open symbols represent data points significantly lower than baseline values. Area of M
max (A) and area of CMEP (B) normalized to the area of the M
max evoked during the same contraction each minute, expressed as a percentage of the baseline value. C, absolute values of cortical voluntary activation measured at baseline (BL) and every 2 min during the fatiguing protocol. At the 10th, 11th, 13th and 15th min, CMEP area was lower in AH compared to CH (#
P < 0.05). Cortical voluntary activation was lower at BL and the 8th and 16th min in AH compared to both N and CH (†
P < 0.05), and at the 14th min in AH relative to CH (#
P < 0.05).
 ), acute hypoxia (AH, ■) and chronic hypoxia (CH,▼). All values are expressed as a percentage of baseline. Open symbols represent data points significantly higher than baseline values. A, cerebral TOI. B, tHb. C, O2Hb. D, HHb. During the second half of the protocol, TOI was higher in all conditions, whereas O2Hb was higher only in AH. For HHb, data were pooled across sessions (no main effect of session, nor a session × time interaction) and post hoc testing on the main effect of time revealed a decrease from the 6th min onward (§
P < 0.05).
), acute hypoxia (AH, ■) and chronic hypoxia (CH,▼). All values are expressed as a percentage of baseline. Open symbols represent data points significantly higher than baseline values. A, cerebral TOI. B, tHb. C, O2Hb. D, HHb. During the second half of the protocol, TOI was higher in all conditions, whereas O2Hb was higher only in AH. For HHb, data were pooled across sessions (no main effect of session, nor a session × time interaction) and post hoc testing on the main effect of time revealed a decrease from the 6th min onward (§
P < 0.05).Comment in
-
The acclimatised spinal cord.J Physiol. 2018 Aug;596(15):2949-2950. doi: 10.1113/JP275552. Epub 2017 Dec 27. J Physiol. 2018. PMID: 29215739 Free PMC article. No abstract available.
-
Differences in muscle performance during fatigue may explain the differences in motoneurone excitability between acute and chronic hypoxia.J Physiol. 2018 Aug;596(15):3425. doi: 10.1113/JP275816. Epub 2018 Mar 5. J Physiol. 2018. PMID: 29436725 Free PMC article. No abstract available.
-
Reply from Luca Ruggiero, Alexandra F. Yacyshyn, Jane Nettleton and Chris J. McNeil.J Physiol. 2018 Aug;596(15):3427. doi: 10.1113/JP275978. Epub 2018 Mar 30. J Physiol. 2018. PMID: 29484649 Free PMC article. No abstract available.
References
-
- Amann M & Kayser B (2009). Nervous system function during exercise in hypoxia. High Alt Med Biol 10, 149–164. - PubMed
-
- Barnholt KE, Hoffman AR, Rock PB, Musa SR, Fulco CS, Braun B, Holloway L, Mazzeo LS, Cymerman A & Friadlander AL (2006). Endocrine responses to acute and chronic high‐altitude exposure (4,300 meters): modulating effects of caloric restriction. Am J Physiol Endocrinol Metab 290, E1078–E1088. - PubMed
-
- Borg G (1982). Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14, 377–381. - PubMed
-
- Caquelard F, Burnet H, Tagliarini F, Cauchy E, Richalet JP & Jammes Y (2000). Effects of prolonged hypobaric hypoxia on human skeletal muscle function and electromyographic events. Clin Sci 98, 329–337. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

