Evaluation of the Pharmacodynamic Effects of the Potassium Binder RDX7675 in Mice
- PMID: 29130735
- PMCID: PMC5987854
- DOI: 10.1177/1074248417741685
Evaluation of the Pharmacodynamic Effects of the Potassium Binder RDX7675 in Mice
Abstract
Introduction: Hyperkalemia is a common complication in patients with heart failure or chronic kidney disease, particularly those who are taking inhibitors of the renin-angiotensin-aldosterone system. RDX7675, the calcium salt of a reengineered polystyrene sulfonate-based resin, is a potassium binder that is being investigated as a novel treatment for hyperkalemia. This study evaluated the pharmacodynamic effects of RDX7675 in mice, compared to 2 current treatments, sodium polystyrene sulfonate (SPS) and patiromer.
Methods: Seven groups of 8 male CD-1 mice were given either standard chow (controls) or standard chow containing 4.0% or 6.6% active moiety of RDX7675, patiromer, or SPS for 72 hours. Stool and urine were collected over the final 24 hours of treatment for ion excretion analyses.
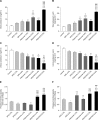
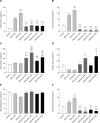
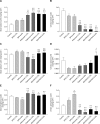
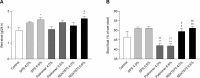
Results: RDX7675 increased stool potassium (mean 24-hour excretion: 4.0%, 9.19 mg; 6.6%, 18.11 mg; both P < .0001) compared with controls (4.47 mg) and decreased urinary potassium (mean 24-hour excretion: 4.0%, 12.05 mg, P < .001; 6.6%, 6.68 mg, P < .0001; vs controls, 20.38 mg). The potassium-binding capacity of RDX7675 (stool potassium/gram of resin: 4.0%, 1.14 mEq/g; 6.6%, 1.32 mEq/g) was greater (all P < .0001) than for patiromer (4.0%, 0.63 mEq/g; 6.6%, 0.48 mEq/g) or SPS (4.0%, 0.73 mEq/g; 6.6% 0.55 mEq/g). RDX7675 and patiromer decreased urinary sodium (mean 24-hour excretion: 0.07-1.38 mg; all P < .001) compared to controls (5.01 mg). In contrast, SPS increased urinary sodium excretion (4.0%, 13.31 mg; 6.6%, 17.60 mg; both P < .0001) compared to controls.
Conclusions: RDX7675 reduced intestinal potassium absorption and had a greater potassium-binding capacity than patiromer or SPS in mice. The calcium-based resins RDX7675 and patiromer reduced intestinal sodium absorption, unlike sodium-based SPS. These results support further studies in humans to confirm the potential of RDX7675 for the treatment of patients with hyperkalemia.
Keywords: RDX7675; hyperkalemia; patiromer; potassium; sodium polystyrene sulfonate.
Conflict of interest statement
Figures
Similar articles
-
Potassium binders for chronic hyperkalaemia in people with chronic kidney disease.Cochrane Database Syst Rev. 2020 Jun 26;6(6):CD013165. doi: 10.1002/14651858.CD013165.pub2. Cochrane Database Syst Rev. 2020. PMID: 32588430 Free PMC article.
-
An Evaluation of the Pharmacodynamics, Safety, and Tolerability of the Potassium Binder RDX7675.J Clin Pharmacol. 2018 Aug;58(8):1035-1043. doi: 10.1002/jcph.1102. Epub 2018 Apr 12. J Clin Pharmacol. 2018. PMID: 29645278
-
Palatability and physical properties of potassium-binding resin RDX7675: comparison with sodium polystyrene sulfonate.Drug Des Devel Ther. 2017 Sep 6;11:2663-2673. doi: 10.2147/DDDT.S143461. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28919716 Free PMC article. Clinical Trial.
-
Potassium-Binding Agents to Facilitate Renin-Angiotensin-Aldosterone System Inhibitor Therapy.Ann Pharmacother. 2016 Jun;50(6):502-10. doi: 10.1177/1060028016640794. Epub 2016 Mar 23. Ann Pharmacother. 2016. PMID: 27009290 Review.
-
Mechanism of Action and Pharmacology of Patiromer, a Nonabsorbed Cross-Linked Polymer That Lowers Serum Potassium Concentration in Patients With Hyperkalemia.J Cardiovasc Pharmacol Ther. 2016 Sep;21(5):456-65. doi: 10.1177/1074248416629549. Epub 2016 Feb 7. J Cardiovasc Pharmacol Ther. 2016. PMID: 26856345 Free PMC article. Review.
Cited by
-
Effects of sodium zirconium cyclosilicate on sodium and potassium excretion in healthy adults: a Phase 1 study.Clin Kidney J. 2020 Dec 13;14(8):1924-1931. doi: 10.1093/ckj/sfaa237. eCollection 2021 Aug. Clin Kidney J. 2020. PMID: 34345416 Free PMC article.
-
Potassium binders for chronic hyperkalaemia in people with chronic kidney disease.Cochrane Database Syst Rev. 2020 Jun 26;6(6):CD013165. doi: 10.1002/14651858.CD013165.pub2. Cochrane Database Syst Rev. 2020. PMID: 32588430 Free PMC article.
-
Current Management of Hyperkalemia in Non-Dialysis CKD: Longitudinal Study of Patients Receiving Stable Nephrology Care.Nutrients. 2021 Mar 15;13(3):942. doi: 10.3390/nu13030942. Nutrients. 2021. PMID: 33804015 Free PMC article.
-
Recent Progresses in Non-Dialysis Chronic Kidney Disease Patients with Hyperkalemia: Outcomes and Therapeutic Strategies.Medicina (Kaunas). 2023 Feb 13;59(2):353. doi: 10.3390/medicina59020353. Medicina (Kaunas). 2023. PMID: 36837554 Free PMC article. Review.
References
-
- Kovesdy CP. Management of hyperkalemia: an update for the internist. Am J Med. 2015;128(12):1281–1287. - PubMed
-
- Desai AS, Swedberg K, McMurray JJV, et al. ; CHARM Program Investigators. Incidence and predictors of hyperkalemia in patients with heart failure: an analysis of the CHARM program. J Am Col Cardiol. 2007;50(20):1959–1966. - PubMed
-
- Pitt B, Bakris GL. New potassium binders for the treatment of hyperkalemia. Hypertension. 2015;66(4):731–738. - PubMed
-
- Brenner BM, Cooper ME, de Zeeuw D, et al. ; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

