Trimethylamine-N-Oxide Instigates NLRP3 Inflammasome Activation and Endothelial Dysfunction
- PMID: 29130962
- PMCID: PMC5828122
- DOI: 10.1159/000484623
Trimethylamine-N-Oxide Instigates NLRP3 Inflammasome Activation and Endothelial Dysfunction
Abstract
Background/aim: Plasma trimethylamine-N-oxide (TMAO), a product of intestinal microbial metabolism of dietary phosphatidylcholine has been recently associated with atherosclerosis and increased risk of cardiovascular diseases (CVD) in rodents and humans. However, the molecular mechanisms of how TMAO induces atherosclerosis and CVD progression are still unclear. The present study tested whether TMAO induces NLRP3 inflammasome formation and activation and thereby contributes to endothelial injury initiating atherogenesis.
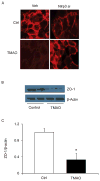
Methods: Inflammasome formation and activation was determined by confocal microscopy, caspase-1 activity was measured by colorimetric assay, IL-1β production was measured using ELISA, cell permeability was determined by microplate reader and ZO-1 expression was determined by western blot analysis and confocal microscopy. In in vivo experiments, TMAO was infused by osmotic pump implantation.
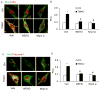
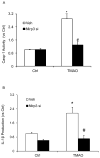
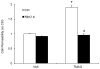
Results: TMAO treatment significantly increased the colocalization of NLRP3 with Asc or NLRP3 with caspase-1, caspase-1 activity, IL-1β production, cell permeability in carotid artery endothelial cells (CAECs) compared to control cells. Pretreatment with caspase-1 inhibitor, WEHD or Nlrp3 siRNA abolished the TMAO-induced inflammasome formation, activation and cell permeability in these cells. In addition, we explored the mechanisms by which TMAO activates NLRP3 inflammasomes. TMAO-induced the activation of NLRP3 inflammasomes was associated with both redox regulation and lysosomal dysfunction. In animal experiments, direct infusion of TMAO in mice with partially ligated carotid artery were found to have increased NLRP3 inflammasome formation and IL-1β production in the intima of wild type mice.
Conclusion: The formation and activation of NLRP3 inflammasomes by TMAO may be an important initiating mechanism to turn on the endothelial inflammatory response leading to endothelial dysfunction.
Keywords: Endothelial cell permeability; Inflammasome; TMAO; Tight junction protein.
© 2017 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors of this manuscript declare that they have no conflicts of interests.
Figures

References
-
- Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
