Molecular function of α7 nicotinic receptors as drug targets
- PMID: 29131336
- PMCID: PMC5978320
- DOI: 10.1113/JP275101
Molecular function of α7 nicotinic receptors as drug targets
Abstract
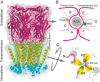
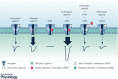
Nicotinic acetylcholine receptors (nAChRs) are pentameric ligand-gated ion channels involved in many physiological and pathological processes. In vertebrates, there are seventeen different nAChR subunits that combine to yield a variety of receptors with different pharmacology, function, and localization. The homomeric α7 receptor is one of the most abundant nAChRs in the nervous system and it is also present in non-neuronal cells. It plays important roles in cognition, memory, pain, neuroprotection, and inflammation. Its diverse physiological actions and associated disorders have made of α7 an attractive novel target for drug modulation. Potentiation of the α7 receptor has emerged as a novel therapeutic strategy for several neurological diseases, such as Alzheimer's and Parkinson's diseases, and inflammatory disorders. In contrast, increased α7 activity has been associated with cancer cell proliferation. The presence of different drug target sites offers a great potential for α7 modulation in different pathological contexts. In particular, compounds that target allosteric sites offer significant advantages over orthosteric agonists due to higher selectivity and a broader spectrum of degrees and mechanisms of modulation. Heterologous expression of α7, together with chaperone proteins, combined with patch clamp recordings have provided important advances in our knowledge of the molecular basis of α7 responses and their potential modulation for pathological processes. This review gives a synthetic view of α7 and its molecular function, focusing on how its unique activation and desensitization features can be modified by pharmacological agents. This fundamental information offers insights into therapeutic strategies.
Keywords: Cys-loop receptors; nicotinic receptors; patch clamp.
© 2017 The Authors. The Journal of Physiology © 2017 The Physiological Society.
Figures


References
-
- Andersen ND, Nielsen BE, Corradi J, Tolosa MF, Feuerbach D, Arias HR & Bouzat C (2016). Exploring the positive allosteric modulation of human α7 nicotinic receptors from a single‐channel perspective. Neuropharmacology 107, 189–200. - PubMed
-
- Arias HR, Gu R‐X, Feuerbach D, Guo B‐B, Ye Y & Wei D‐Q (2011). Novel positive allosteric modulators of the human α7 nicotinic acetylcholine receptor. Biochemistry 50, 5263–5278. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

