Iodide Binding in Sodium-Coupled Cotransporters
- PMID: 29131623
- PMCID: PMC5744185
- DOI: 10.1021/acs.jcim.7b00521
Iodide Binding in Sodium-Coupled Cotransporters
Abstract
Several apical iodide translocation pathways have been proposed for iodide efflux out of thyroid follicular cells, including a pathway mediated by the sodium-coupled monocarboxylate transporter 1 (SMCT1), which remains controversial. Herein, we evaluate structural and functional similarities between SMCT1 and the well-studied sodium-iodide symporter (NIS) that mediates the first step of iodide entry into the thyroid. Free-energy calculations using a force field with electronic polarizability verify the presence of a conserved iodide-binding pocket between the TM2, TM3, and TM7 segments in hNIS, where iodide is coordinated by Phe67, Gln72, Cys91, and Gln94. We demonstrate the mutation of residue Gly93 of hNIS to a larger amino acid expels the side chain of a critical tryptophan residue (Trp255) into the interior of the binding pocket, partially occluding the iodide binding site and reducing iodide affinity, which is consistent with previous reports associating mutation of this residue with iodide uptake deficiency and hypothyroidism. Furthermore, we find that the position of Trp255 in this hNIS mutant mirrors that of Trp253 in wild-type hSMCT1, where a threonine (Thr91) occupies the position homologous to that occupied by glycine in wild-type hNIS (Gly93). Correspondingly, mutation of Thr91 to glycine in hSMCT1 makes the pocket structure more like that of wild-type hNIS, increasing its iodide affinity. These results suggest that wild-type hSMCT1 in the inward-facing conformation may bind iodide only very weakly, which may have implications for its ability to transport iodide.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




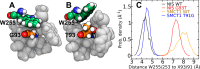

References
-
- Werner S. C.; Ingbar S. H.; Braverman L. E.; Utiger R. D.. Werner & Ingbar’s the Thyroid: A Fundamental and Clinical Text; Lippincott Williams & Wilkins, 2005.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

