Secretome of Aggregated Embryonic Stem Cell-Derived Mesenchymal Stem Cell Modulates the Release of Inflammatory Factors in Lipopolysaccharide-Induced Peripheral Blood Mononuclear Cells
- PMID: 29132205
- PMCID: PMC5949126
- DOI: 10.22034/ibj.22.4.237
Secretome of Aggregated Embryonic Stem Cell-Derived Mesenchymal Stem Cell Modulates the Release of Inflammatory Factors in Lipopolysaccharide-Induced Peripheral Blood Mononuclear Cells
Abstract
Background: Bone marrow mesenchymal stem cells (BM-MSCs) have emerged as a potential therapy for various inflammatory diseases. Because of some limitations, several recent studies have suggested the use of embryonic stem cell-derived MSCs (ESC-MSCs) as an alternative for BM-MSCs. Some of the therapeutic effects of the ESC-MSCs are related to the secretion of a broad array of cytokines and growth factors, known as secretome. Harnessing this secretome for therapeutic applications requires the optimization of production of secretary molecules. It has been shown that aggregation of MSCs into 3D spheroids, as a preconditioning strategy, can enhance immunomodulatory potential of such cells. In this study, we investigated the effect of secretome derived from human ESC-MSCs (hESC-MSCs) spheroids on secretion of IL-1β, IL-10, and tumor necrosis factor α (TNF-α) from lipopolysaccharide (LPS)-induced peripheral blood mononuclear cells (PBMCs).
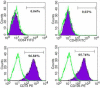
Methods: In the present study, after immunophenotyping and considering mesodermal differentiation of hESC-MSCs, the cells were non-adherently grown to prepare 3D aggregates, and then conditioned medium or secretome was extracted from the cultures. Afterwards, the anti-inflammatory effects of the secretome were assessed in an in vitro model of inflammation.
Results: Results from this study showed that aggregate-prepared secretome from hESC-MSCs was able to significantly decrease the secretion of TNF-α (301.7 ± 5.906, p < 0.0001) and IL-1β (485.2 ± 48.38, p < 0.001) from LPS-induced PBMCs as the indicators of inflammation, in comparison with adherent culture-prepared secretome (TNF-α: 166.6 ± 8.04, IL-1β: 125.2 ± 2.73).
Conclusion: Our study indicated that cell aggregation can be an appropriate strategy to increase immunomodulatory characteristics of hESC-MSCs.
Keywords: Cell aggregation; Embryonic stem cells; Mesenchymal stem cells; Inflammation; Mononuclear leukocyte.
Conflict of interest statement
Figures



References
-
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. - PubMed
-
- Ben-Ami E, Berrih-Aknin S, Miller A. Mesenchymal stem cells as an immunomodulatory therapeutic strategy for autoimmune diseases. Autoimmunity reviews. 2011;10(7):410–415. - PubMed
-
- Bianco P. Mesenchymal stem cells. Annual review of cell and developmental biology. 2014;30:677–704. - PubMed
-
- Hematti P. Human embryonic stem cell-derived mesenchymal progenitors:an overview. Methods in molecular biology. 2011;690:163–174. - PubMed
LinkOut - more resources
Full Text Sources
