Chemical Probes for Redox Signaling and Oxidative Stress
- PMID: 29132214
- PMCID: PMC6391619
- DOI: 10.1089/ars.2017.7408
Chemical Probes for Redox Signaling and Oxidative Stress
Abstract
Significance: Cellular reactive oxygen species (ROS) mediate redox signaling cascades that are critical to numerous physiological and pathological processes. Analytical methods to monitor cellular ROS levels and proteomic platforms to identify oxidative post-translational modifications (PTMs) of proteins are critical to understanding the triggers and consequences of redox signaling. Recent Advances: The prevalence and significance of redox signaling has recently been illuminated through the use of chemical probes that allow for sensitive detection of cellular ROS levels and proteomic dissection of oxidative PTMs directly in living cells.
Critical issues: In this review, we provide a comprehensive overview of chemical probes that are available for monitoring ROS and oxidative PTMs, and we highlight the advantages and limitations of these methods.
Future directions: Despite significant advances in chemical probes, the low levels of cellular ROS and low stoichiometry of oxidative PTMs present challenges for accurately measuring the extent and dynamics of ROS generation and redox signaling. Further improvements in sensitivity and ability to spatially and temporally control readouts are essential to fully illuminate cellular redox signaling.
Keywords: cysteine oxidation; oxidative stress; redox proteomics; redox sensors.
Figures

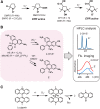
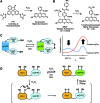


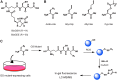
Similar articles
-
Redox sensing by proteins: oxidative modifications on cysteines and the consequent events.Antioxid Redox Signal. 2012 Apr 1;16(7):649-57. doi: 10.1089/ars.2011.4313. Epub 2011 Dec 19. Antioxid Redox Signal. 2012. PMID: 21967570 Review.
-
Analysis of Cysteine Redox Post-Translational Modifications in Cell Biology and Drug Pharmacology.Methods Mol Biol. 2017;1558:191-212. doi: 10.1007/978-1-4939-6783-4_9. Methods Mol Biol. 2017. PMID: 28150239
-
Redox proteomics: from bench to bedside.Adv Exp Med Biol. 2014;806:301-17. doi: 10.1007/978-3-319-06068-2_13. Adv Exp Med Biol. 2014. PMID: 24952188 Review.
-
Mass spectrometry and redox proteomics: applications in disease.Mass Spectrom Rev. 2014 Jul-Aug;33(4):277-301. doi: 10.1002/mas.21374. Epub 2013 Sep 30. Mass Spectrom Rev. 2014. PMID: 24930952 Review.
-
Differential redox proteomics allows identification of proteins reversibly oxidized at cysteine residues in endothelial cells in response to acute hypoxia.J Proteomics. 2012 Sep 18;75(17):5449-62. doi: 10.1016/j.jprot.2012.06.035. Epub 2012 Jul 16. J Proteomics. 2012. PMID: 22800641
Cited by
-
Triterpenoids as Reactive Oxygen Species Modulators of Cell Fate.Chem Res Toxicol. 2022 Apr 18;35(4):569-584. doi: 10.1021/acs.chemrestox.1c00428. Epub 2022 Mar 21. Chem Res Toxicol. 2022. PMID: 35312315 Free PMC article. Review.
-
Dual-Reactivity trans-Cyclooctenol Probes for Sulfenylation in Live Cells Enable Temporal Control via Bioorthogonal Quenching.J Am Chem Soc. 2019 Jul 17;141(28):10932-10937. doi: 10.1021/jacs.9b01164. Epub 2019 Jul 9. J Am Chem Soc. 2019. PMID: 31246462 Free PMC article.
-
Getting the Message? Native Reactive Electrophiles Pass Two Out of Three Thresholds to be Bona Fide Signaling Mediators.Bioessays. 2018 May;40(5):e1700240. doi: 10.1002/bies.201700240. Epub 2018 Mar 30. Bioessays. 2018. PMID: 29603288 Free PMC article. Review.
-
Sambucus nigra-Lyophilized Fruit Extract Attenuated Acute Redox-Homeostatic Imbalance via Mutagenic and Oxidative Stress Modulation in Mice Model on Gentamicin-Induced Nephrotoxicity.Pharmaceuticals (Basel). 2025 Jan 13;18(1):85. doi: 10.3390/ph18010085. Pharmaceuticals (Basel). 2025. PMID: 39861148 Free PMC article.
-
Redox Mechanisms in Neurodegeneration: From Disease Outcomes to Therapeutic Opportunities.Antioxid Redox Signal. 2019 Apr 10;30(11):1450-1499. doi: 10.1089/ars.2017.7321. Epub 2018 May 4. Antioxid Redox Signal. 2019. PMID: 29634350 Free PMC article. Review.
References
-
- Abo M, Minakami R, Miyano K, Kamiya M, Nagano T, Urano Y, and Sumimoto H. Visualization of phagosomal hydrogen peroxide production by a novel fluorescent probe that is localized via SNAP-tag labeling. Anal Chem 86: 5983–5990, 2014 - PubMed
-
- Abo M, Urano Y, Hanaoka K, Terai T, Komatsu T, and Nagano T. Development of a highly sensitive fluorescence probe for hydrogen peroxide. J Am Chem Soc 133: 10629–10637, 2011 - PubMed
-
- Abo M. and Weerapana E. A caged electrophilic probe for global analysis of cysteine reactivity in living cells. J Am Chem Soc 137: 7087–7090, 2015 - PubMed
-
- Afzal M, Matsugo S, Sasai M, Xu B, Aoyama K, and Takeuchi T. Method to overcome photoreaction, a serious drawback to the use of dichlorofluorescin in evaluation of reactive oxygen species. Biochem Biophys Res Commun 304: 619–624, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

