In Vivo Molecular Electron Paramagnetic Resonance-Based Spectroscopy and Imaging of Tumor Microenvironment and Redox Using Functional Paramagnetic Probes
- PMID: 29132215
- PMCID: PMC5910053
- DOI: 10.1089/ars.2017.7329
In Vivo Molecular Electron Paramagnetic Resonance-Based Spectroscopy and Imaging of Tumor Microenvironment and Redox Using Functional Paramagnetic Probes
Abstract
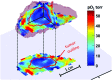
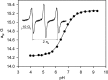
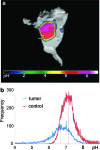
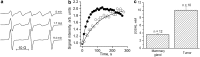
Significance: A key role of the tumor microenvironment (TME) in cancer progression, treatment resistance, and as a target for therapeutic intervention is increasingly appreciated. Among important physiological components of the TME are tissue hypoxia, acidosis, high reducing capacity, elevated concentrations of intracellular glutathione (GSH), and interstitial inorganic phosphate (Pi). Noninvasive in vivo pO2, pH, GSH, Pi, and redox assessment provide unique insights into biological processes in the TME, and may serve as a tool for preclinical screening of anticancer drugs and optimizing TME-targeted therapeutic strategies. Recent Advances: A reasonable radiofrequency penetration depth in living tissues and progress in development of functional paramagnetic probes make low-field electron paramagnetic resonance (EPR)-based spectroscopy and imaging the most appropriate approaches for noninvasive assessment of the TME parameters.
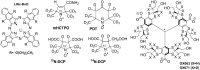
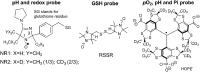
Critical issues: Here we overview the current status of EPR approaches used in combination with functional paramagnetic probes that provide quantitative information on chemical TME and redox (pO2, pH, redox status, Pi, and GSH). In particular, an application of a recently developed dual-function pH and redox nitroxide probe and multifunctional trityl probe provides unsurpassed opportunity for in vivo concurrent measurements of several TME parameters in preclinical studies. The measurements of several parameters using a single probe allow for their correlation analyses independent of probe distribution and time of measurements.
Future directions: The recent progress in clinical EPR instrumentation and development of biocompatible paramagnetic probes for in vivo multifunctional TME profiling eventually will make possible translation of these EPR techniques into clinical settings to improve prediction power of early diagnostics for the malignant transition and for future rational design of TME-targeted anticancer therapeutics. Antioxid. Redox Signal. 28, 1365-1377.
Keywords: electron paramagnetic resonance; paramagnetic probes; tumor acidosis; tumor hypoxia; tumor microenvironment; tumor redox.
Conflict of interest statement
No competing financial interests exist.
Figures
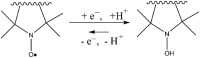





Similar articles
-
In Vivo Electron Paramagnetic Resonance: Radical Concepts for Translation to the Clinical Setting.Antioxid Redox Signal. 2018 May 20;28(15):1341-1344. doi: 10.1089/ars.2017.7472. Epub 2018 Feb 12. Antioxid Redox Signal. 2018. PMID: 29304554 Free PMC article.
-
In Vivo EPR Assessment of pH, pO2, Redox Status, and Concentrations of Phosphate and Glutathione in the Tumor Microenvironment.J Vis Exp. 2018 Mar 16;(133):56624. doi: 10.3791/56624. J Vis Exp. 2018. PMID: 29608148 Free PMC article.
-
In Vivo Electron Paramagnetic Resonance Molecular Profiling of Tumor Microenvironment upon Tumor Progression to Malignancy in an Animal Model of Breast Cancer.Mol Imaging Biol. 2024 Jun;26(3):424-434. doi: 10.1007/s11307-023-01847-0. Epub 2023 Aug 23. Mol Imaging Biol. 2024. PMID: 37610610 Free PMC article.
-
Pulsed Electron Paramagnetic Resonance Imaging: Applications in the Studies of Tumor Physiology.Antioxid Redox Signal. 2018 May 20;28(15):1378-1393. doi: 10.1089/ars.2017.7391. Epub 2018 Jan 9. Antioxid Redox Signal. 2018. PMID: 29130334 Free PMC article. Review.
-
Janus-faced tumor microenvironment and redox.Antioxid Redox Signal. 2014 Aug 10;21(5):723-9. doi: 10.1089/ars.2014.5864. Epub 2014 Mar 4. Antioxid Redox Signal. 2014. PMID: 24512276 Free PMC article. Review.
Cited by
-
Dextran-conjugated tetrathiatriarylmethyl radicals as biocompatible spin probes for EPR spectroscopy and imaging.Bioorg Med Chem Lett. 2019 Jul 15;29(14):1756-1760. doi: 10.1016/j.bmcl.2019.05.017. Epub 2019 May 13. Bioorg Med Chem Lett. 2019. PMID: 31129052 Free PMC article.
-
In Vivo Electron Paramagnetic Resonance: Radical Concepts for Translation to the Clinical Setting.Antioxid Redox Signal. 2018 May 20;28(15):1341-1344. doi: 10.1089/ars.2017.7472. Epub 2018 Feb 12. Antioxid Redox Signal. 2018. PMID: 29304554 Free PMC article.
-
A combined positron emission tomography (PET)-electron paramagnetic resonance imaging (EPRI) system: initial evaluation of a prototype scanner.Phys Med Biol. 2018 May 16;63(10):105010. doi: 10.1088/1361-6560/aabfa1. Phys Med Biol. 2018. PMID: 29676283 Free PMC article.
-
Improving combination therapies: targeting A2B-adenosine receptor to modulate metabolic tumor microenvironment and immunosuppression.J Natl Cancer Inst. 2023 Nov 8;115(11):1404-1419. doi: 10.1093/jnci/djad091. J Natl Cancer Inst. 2023. PMID: 37195421 Free PMC article.
-
Sensitive simultaneous measurements of oxygenation and extracellular pH by EPR using a stable monophosphonated trityl radical and lithium phthalocyanine.Free Radic Biol Med. 2024 Mar;213:11-18. doi: 10.1016/j.freeradbiomed.2024.01.012. Epub 2024 Jan 11. Free Radic Biol Med. 2024. PMID: 38218552 Free PMC article.
References
-
- Ardenkjaer-Larsen JH, Laursen I, Leunbach I, Ehnholm G, Wistrand LG, Petersson JS, and Golman K. EPR and DNP properties of certain novel single electron contrast agents intended for oximetric imaging. J Magn Reson 133: 1–12, 1998 - PubMed
-
- Bacic G, Nilges MJ, Magin RL, Walczak T, and Swartz HM. In vivo localized ESR spectroscopy reflecting metabolism. Magn Reson Med 10: 266–272, 1989 - PubMed
-
- Backer JM, Budker VG, Eremenko SI, and Molin YN. Detection of the kinetics of biochemical reactions with oxygen using exchange broadening in the ESR spectra of nitroxide radicals. Biochim Biophys Acta 460: 152–156, 1977 - PubMed
-
- Barriga-Gonzalez G, Olea-Azar C, Zuniga-Lopez MC, Folch-Cano C, Aguilera-Venegas B, Porcal W, Gonzalez M, and Cerecetto H. Spin trapping: An essential tool for the study of diseases caused by oxidative stress. Curr Top Med Chem 15: 484–495, 2015 - PubMed
-
- Bauer G. Tumor cell-protective catalase as a novel target for rational therapeutic approaches based on specific intercellular ROS signaling. Anticancer Res 32: 2599–2624, 2012 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

