Oxidative Stress in HIV Infection and Alcohol Use: Role of Redox Signals in Modulation of Lipid Rafts and ATP-Binding Cassette Transporters
- PMID: 29132227
- PMCID: PMC5743035
- DOI: 10.1089/ars.2016.6830
Oxidative Stress in HIV Infection and Alcohol Use: Role of Redox Signals in Modulation of Lipid Rafts and ATP-Binding Cassette Transporters
Abstract
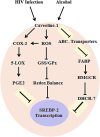
Aims: Human immunodeficiency virus (HIV) infection induces oxidative stress and alcohol use accelerates disease progression, subsequently causing immune dysfunction. However, HIV and alcohol impact on lipid rafts-mediated immune dysfunction remains unknown. In this study, we investigate the modulation by which oxidative stress induces reactive oxygen species (ROS) affecting redox expression, lipid rafts caveiloin-1, ATP-binding cassette (ABC) transporters, and transcriptional sterol regulatory element-binding protein (SREBP) gene and protein modification and how these mechanisms are associated with arachidonic acid (AA) metabolites in HIV positive alcohol users, and how they escalate immune dysfunction.
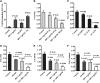
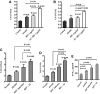
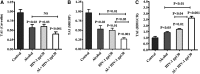
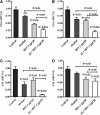
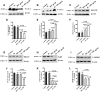
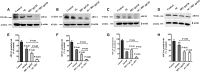
Results: In both alcohol using HIV-positive human subjects and in vitro studies of alcohol with HIV-1 gp120 protein in peripheral blood mononuclear cells, increased ROS production significantly affected redox expression in glutathione synthetase (GSS), super oxide dismutase (SOD), and glutathione peroxidase (GPx), and subsequently impacted lipid rafts Cav-1, ABC transporters ABCA1, ABCG1, ABCB1, and ABCG4, and SREBP transcription. The increased level of rate-limiting enzyme 3-hydroxy-3-methylglutaryl HMG-CoA reductase (HMGCR), subsequently, inhibited 7-dehydrocholesterol reductase (DHCR-7). Moreover, the expression of cyclooxygenase-2 (COX-2) and lipoxygenase-5 (5-LOX) mRNA and protein modification tentatively increased the levels of prostaglandin E2 synthases (PGE2) in plasma when compared with either HIV or alcohol alone.
Innovation: This article suggests for the first time that the redox inhibition affects lipid rafts, ABC-transporter, and SREBP transcription and modulates AA metabolites, serving as an important intermediate signaling network during immune cell dysfunction in HIV-positive alcohol users.
Conclusion: These findings indicate that HIV infection induces oxidative stress and redox inhibition, affecting lipid rafts and ABC transports, subsequently upregulating AA metabolites and leading to immune toxicity, and further exacerbation with alcohol use. Antioxid. Redox Signal. 28, 324-337.
Keywords: ABC transporters; HIV; alcohol; immune cells; lipid rafts; redox expression.
Conflict of interest statement
No competing financial interests exist.
Figures
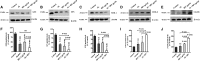
References
-
- Bagasra O, Bachman SE, Jew L, Tawadros R, Cater J, Boden G, Ryan I, and Pomerantz RJ. Increased human immunodeficiency virus type 1 replication in human peripheral blood mononuclear cells induced by ethanol: potential immunopathogenic mechanisms. J Infect Dis 173: 550–558, 1996 - PubMed
-
- Bagasra O, Kajdacsy-Balla A, Lischner HW, and Pomerantz RJ. Alcohol intake increases human immunodeficiency virus type 1 replication in human peripheral blood mononuclear cells. J Infect Dis 167: 789–797, 1993 - PubMed
-
- Balla AK, Lischner HW, Pomerantz RJ, and Bagasra O. Human studies on alcohol and susceptibility to HIV infection. Alcohol 11: 99–103, 1994 - PubMed
-
- Bautista AP. and Wang E. Chronic ethanol intoxication enhances the production of cytokine-induced neutrophil chemoattractant and macrophage inflammatory protein-2 by hepatocytes after human immunodeficiency virus-1 glycoprotein 120 vaccination. Alcohol 24: 35–44, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

